Investigation: Wave mechanical View of the Hydrogen Atom
advertisement

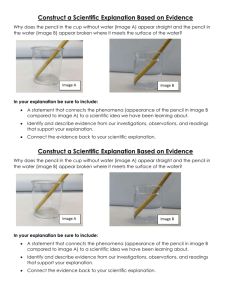
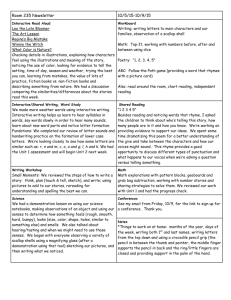
Section 5.1 Lab Honors Chemistry Quantum Mechanical Model of the Atom Purpose: To be able to visualize the quantum mechanical model of an atom Procedure 1. Put your target on the floor. 2. Drop your pencil from a height of about 1-meter (waist high) onto the target. Try to hit the CENTER by aiming at the center of the target. Repeat for a total of 100 trials. Data Table: Count the number of marks in each numbered area of the target. Record in your data table as follows: Area 1 2 3 4 5 6 Number of Marks Post Lab Questions: 1. Before you dropped the pencil, could you predict exactly where it would strike the target? 2. If you could not predict the exact spot where the pencil would hit the target, could you predict the area within which it would hit? 3. Did your predictions change as you made more drops? 4. What does probability mean? 5. Make a rough sketch of a graph (number of marks on the y-axis vs. area number on the x-axis) using the data collected. Sketch the graph in your lab notebook. 6. From your graph, which target area had the highest probability of being hit by the pencil? 7. How is the idea of electron clouds different from Bohr’s idea of atomic orbits? Conclusion: If you were to repeat this experiment, would you expect similar results?