Puetzer Abstract
advertisement

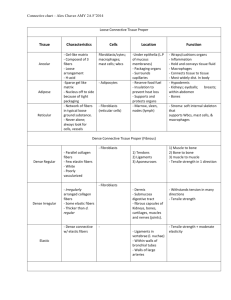
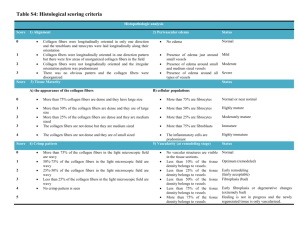
Investigation and Development of Molecular Crosslinking in Collagen Fibers for Mechanical Integrity of Engineered Tissues Jennifer Puetzer and Molly Stevens Departments of Materials and Bioengineering, Imperial College London, UK Collagen fibers provide the tensile strength in multiple tissues, particularly orthopaedic tissues. Tissue engineers are currently striving to create mechanically similar replacements of these tissues; however these attempts often fail to create organized collagen fibers that are essential to long-term mechanical success. It is believed the specific organization of collagen, including fiber density, diameter, orientation, and degree of crosslinking are fundamental to the mechanical integrity of engineered tissues. Previously, I have developed native sized and aligned collagen fibers with cellseeded high density collagen gels; however the tensile properties of these constructs are orders of magnitude less than native tissue, suggesting the need for further crosslinking. The molecular mechanism of fiber assembly is still poorly understood. The objective of this study is to use Raman micro-spectroscopy and Fourier transform infrared spectroscopy (FTIR) to gain valuable molecular insight into cellular development of collagen fibers and use these insights to further develop collagen fibers for use in orthopaedic tissue engineering applications. Cell-seeded high density collagen gels will be cultured for up to 8 weeks using boundary conditions to force the development of aligned collagen fibers. Raman and FTIR will be used to determine molecular changes with fiber development and results will be compared to tensile properties. Further, the effect of temporal treatment with nonenzymatic and enzymatic crosslinking agents will be evaluated. The completion of this project will provide new insight into how collagen fibers develop chemically in vitro and the effect of crosslinkers at various stages of fiber development. Furthermore, it will provide a treatment method for developing the most organized collagen scaffolds to date and a rapid, non-destructive Raman technique for analyzing collagen fiber development in countless engineered tissues.