Chapter 2 Study Guide - Warren Hills Regional School District
advertisement

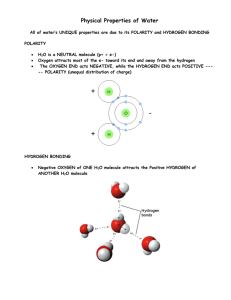
Chapter 2--Chemistry of Life-- Study Guide Topics discussed: Chemistry of life (basics) pH Properties of Water Biochemistry Enzymes 1. What is cohesion? Attraction between molecules of the same substance. Why H2O forms beads on a smooth surface 2. What is adhesion? Attraction between molecules of different substances; in plants: attraction between unlike molecules Water properties. A water molecule is held together by strong, polar covalent bonds between oxygen and hydrogen atoms. The partially charged regions of a polar water molecule are attracted to oppositely charged parts of neighboring molecules. Each molecule can form weak hydrogen bonds to multiple partners, conferring water unique properties. Cohesive – because of hydrogen bonding 3. Why does ice float on water? Because it is slightly less dense than cold H2O. 4. What are all of the properties of water that make it so essential to life? It is neutral, polar & a solvent (Versatile solvent for hydrophilic substances). It is found as a gas, liquid & a solid in nature H2O resists temperature change: o The high specific heat capacity of H2O helps the earth's temperature remain moderate since H2O traps heat during the day and releases it slowly at night. o H2O also has a very high boiling point, meaning that liquid H2O turns into H2O vapor at a higher temperature (212°F) than would be expected due to the size and weight of the molecule. The high boiling point of H2O is due to the hydrogen bonds which tend to hold H2O molecules together, preventing them from breaking apart and entering the gaseous state. High specific heat. H2O expands when it freezes: o The density of H2O, once again, is a special case. H2O is most dense at 39°F, and as it cools or warms from this temperature, the H2O expands slightly. This means that ice is slightly less dense than cold H2O, which is why ice floats on the surface of bodies of H2O. The floating ice slows the freezing process by insulating the H2O underneath, which contributes to the moderate temperatures on earth. In addition, the layer of ice prevents many lakes from freezing solid, allowing fish and other organisms to survive under the ice. o The changing density of water at different temperatures is also responsible for turnover. Turnover occurs when the H2O on the surface of a lake cools in the fall. Eventually, this cold H2O will become denser than the warmer H2O beneath, so the cold H2O will sink to the bottom and the warm H2O will rise to the surface. When lakes are used as the H2O source for H2O treatment plants, turnover can cause abrupt changes in the quality of the raw H2O. In a body of H2O, hydrogen bonds between H2O molecules are constantly pulling the molecules in many different directions. However, at the water's surface, the molecules are only being pulled from side to side and down, with no hydrogen bonds pulling them upwards. This results in a skin of H2O at the surface in which the molecules are held together very tightly. Surface tension is a measurement of the amount of force required to break this skin on the surface of H2O. Other liquids have a surface tension as well, but the surface tension in H2O is quite strong due to the hydrogen bonds. The pictures below show some examples of the results of water's strong surface tension. Surface tension is what holds drops of H2O together in a round shape. Surface tension allows both H2O striders and paperclips to float on H2O even though they are denser than the H2O. In addition, surface tension allows you to fill a cup slightly over the brim with H2O. Service tension is responsible for capillary action. 5. What is capillary action? Occurs when H2O climbs upward through a small space, defying gravity due to the forces of adhesion and surface tension. The image below shows one example of capillary action - a narrow straw was placed in a cup of H2O and the H2O crept upwards through the straw. What causes the movement of H2O during capillary action? The first factor is adhesion, the attraction between H2O and another object. In this case, adhesion attracted the H2O within the straw to the surface of the straw. Molecules of H2O which came in contact with the straw tended to move upward along the inside of the straw, as shown below: Water's surface tension is so strong that, as H2O is pulled upward along the straw's walls, the H2O in between tends to be pulled upward also. The downward pull of gravity prevents the central H2O from rising quite as high as the H2O which is adhered to the straw, so the result is a meniscus, as shown in the first picture in this section. Capillary action is important in moving H2O upwards through small spaces. Plants depend on capillary action to move H2O upward from the roots to the leaves. In the soil, capillary action also tends to move H2O upward between the soil particles. 6. Differentiate between atoms, elements, molecules, and compounds. If drawing would help, please do so! Atoms are the basic units of matter. They are the smallest components that maintain the chemical properties of an element, which is a substance that cannot be broken down into simpler material. Atoms have specific properties that will determine their chemical and physical nature. One of these properties is their atomic mass. Atom – basic unit of matter; element – pure substance consisting entirely of one type of atom; molecule – structure that results when atoms are joined together by covalent bonds, smallest unit of most compounds; compounds – substance formed by the chemical combination of 2 or more elements in definite proportions. 7. What does the atomic number represent? The number of protons in the nucleus of an atom, which determines the chemical properties of an element and its place in the periodic table. Atomic mass? Atomic mass is roughly equal to the sum of the individual particle masses of an atom. Atoms have three basic components: protons (positively charged particles), neutrons (non- charged particles), and electrons (negatively charged particles). Protons and neutrons are the larger particles, and are found in the nucleus, or core, of the atom. Atomic mass is typically calculated by adding the number of protons and neutrons together, ignoring the electrons because of their small size. 8. What is an isotope? One of several nuclides having the same number of protons in their nuclei and hence having the same atomic number, but differing in the number of neutrons and therefore, in the mass number. Almost identical chemical properties exist between isotopes of a particular element. An isotope is a variant of an element, with a different # of neutrons than is typical. This makes a nucleus unstable. Give an example of one we discussed. carbon-12, carbon-13 and carbon-14 are three isotopes of the element carbon with mass numbers 12, 13 and 14 respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons, so that the neutron numbers of these isotopes are 6, 7 and 8 respectively. 9. What are the subatomic particles? Be sure to mention their charge and where they are located. Protons – positive – in the nucleus Neutrons – neutral – in the nucleus Electrons – negative – surrounding nucleus 10. What is pH (potential of hydrogen) a measure of (DON’T TELL ME ACIDITY!!) Measure of the acidity or basicity of an aqueous solution 11. The pH scale goes from 0 to 14. 12. What classifies as an acid? Neutral? A base? Acids have higher amounts of hydrogen ions (H+) Ex: HCL (anything from 0-6 on scale) Ex: milk=6, vinegar, lemon juice, battery acid Bases have higher amounts of hydroxide ions (OH-) scale) Ex: lye, ammonia, milk of magnesia, baking soda Neutral has a pH of 7 Ex: pure H2O 13. Which pH is more alkaline: 8 or 10? 10 Ex: NaOH (anything from 7-14 on 14. What does it mean to say water is a polar molecule? (explain why it is) H2O is polar because there is an uneven distribution of electrons between the oxygen and hydrogen atoms. The oxygen end of the molecule has a slight negative charge & the hydrogen end has a slight positive charge. 15. What are the two main types of bonds? Differentiate between the two. Covalent – bond formed by electrons being shared between atoms Most compounds have combinations of ionic and covalent bonds. Ionic – bond formed when one or more electrons are transferred from one atom to another 16. What kind of bond does a molecule of oxygen have? What kind of bond does NaCl have? Oxygen – covalent NaCl – ionic 17. Compare polar covalent bonds to nonpolar covalent bonds. Nonpolar won’t dissolve in H2O Polar A bond between 2 nonmetal atoms that have different electronegativities and therefore have unequal sharing of the bonding electron pair Ex: H2O 18. What type of bond is found in between the atoms of a water molecule? Single covalent bond. What type of bond is found between two water molecules? Hydrogen bond 19. What is a solution? Solvent? Solute? Solution – a mixture of 2 or more substances in which the molecules of the substances are evenly mixed Solvent – substance in which the solute dissolves Solute – substance that is dissolved 20. What are the 6 most abundant elements in the human body? Carbon, hydrogen, nitrogen, oxygen, calcium, potassium, and sulfur 21. What is an organic compound? Made mostly of carbon, and some hydrogen (Four main classes – carbohydrates, lipids, proteins, nucleic acids) 22. What is an element? A chemical element is a pure substance that consists entirely of one type of atom. 23. Describe why carbon is able to bond so easily with different atoms. (use the term valence electrons in your answer) The carbon atom has four valence (outermost) electrons. Because of this unique configuration, it is easier for the carbon atom to share its four electrons with another atom or atoms than to lose or gain four electrons. Because each carbon is identical, they all have four valence electrons, so they can easily bond with other carbon atoms to form long chains or rings. In fact, a carbon atom can bond with another carbon atom two or three times to make double and triple covalent bonds between two carbon atoms. Long chains of carbon atoms with double and triple bonds are quite common in biology. Carbon's tendency toward covalent bonding with itself generates three unique characteristics that create a vast array of compounds, including those necessary to construct and support life: • • • The single bond that connects carbon atoms to carbon atoms is quite strong, so the subsequent long chains and ring structures are not fragile. The carbon-carbon covalent bonding pattern satisfies the Octet rule, making carbon compounds unwilling to react. Because carbon has four valence electrons and needs eight to satisfy the Octet rule, it can bond with up to four additional atoms, creating countless compound possibilities. 24. Fill in the chart below about the four organic molecules: Polymer Monomer CARBOHYDRATE LIPIDS PROTEINS NUCLEIC ACIDS Examples Monosaccharaides (simple sugars) Functions Quick energy, produced during cell respiration, structural support Triglycerides Glycerol & fatty acids Used as stored energy Major components in cell membrane Cholesterol Plant pigments like chlorophyll Building blocks of cell components, produced from DNA material, form bones & muscles, fight disease, makes up enzymes Insulin, Actin, myosin Amino acids nucleotides Carry DNA & RNA material; store & transmit hereditary or genetic information Glucose Galactose Fructose DNA RNA ATP 25. What is a catalyst? A substance that speeds up the rate of a chemical reaction. Catalysts are substances that reduce the activation energy of a chemical reaction, facilitating it or making it energetically viable. 26. What are enzymes? Proteins that act as biological catalysts are enzymes. What type of organic molecule are they? Enzymes are protein molecules. What is their function? Cells use enzymes to speed up chemical reactions that take place in cells. 27. What does it mean if something denatures? It can’t carry out its cellular function. (Enzyme shape is changed thereby denaturing the protein). 28. What can cause denaturation? Excessive heat, a change in neutral pH (because they change the shape of enzymes & their active sites. 29. What is a monomer? Monomer – small unit that can join together with other small units to form polymers A polymer? Polymer – large compound formed from combinations of many monomers 30. What is the term used to describe how much energy is needed to start a chemical reaction? Activation energy What is the effect of an enzyme on this amount of energy? An enzyme may accelerate a reaction by a factor of 1010 , making it 10 billion times faster. 31. A chemical reaction takes place when (reactants) turn into (products). 32. Describe why the enzyme-substrate relationship is called a lock and key model. The enzyme has spatial binding sites for the attachment of its substrate. These sites are called activation centers of the enzyme. Substrates bind to these centers forming the enzyme-substrate complex. In the lock and key model the enzyme has a region with specific spatial conformation for the binding of the substrate. In the induced fit model the binding of the substrate induces a change in the spatial configuration of the enzyme for the substrate to fit.