Chemistry 102 -2-credit hour course meeting 2 classes (1:15 hr.) per week
Instructor: Dr. Upali Siriwardane
E-mail: Upali@latech.edu;
Textbook:
Principles of Chemistry: A Molecular Approach, 2nd Edition-Nivaldo J. Tro - Pearson Prentice Hall: and
also purchase the Mastering Chemistry with Pearson online web learning component, the Mastering
Chemistry. The study guide and solution manual also could be purchased if you need more help.
Specific Course Information:
Catalogue Description:
Rates of reaction, study of chemical equilibria including those involving acids, bases, sparingly soluble salts and
complex ions, thermodynamics of equilibrium and introductory electrochemistry.
Prerequisite: CHEM 101
Required courses in the Program: (as per table 5-10).
Specific goals for the course
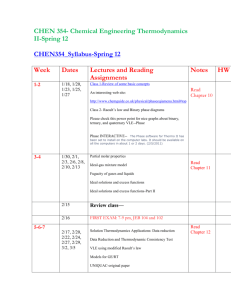
Chapter 13
a) Recognize, define and apply the concepts of reaction rate and rate laws and rate constants, use the
integrated rate method to obtain orders and rate constants and half-life.
b) Recognize, define and apply the nanoscale concept of reaction mechanism, elementary reactions,
unimolecular and bimolecular elementary reactions, energy profile diagrams, collision theory, transition
state theory, effect of temperature, effect of catalysts and activation energy.
c) Recognize, define and apply Arrhenius law, temperature dependence of rate constant, and calculation of
activation energy.
Chapter 14
a) Recognize, define and apply the concepts of equilibrium, the dynamic nature of equilibrium causing
changes in concentrations in approaching equilibrium, and equilibrium constant expressions K and Kp
for a chemical equations.
b) Recognize, define and apply the concepts of equilibrium constant in K and Kp form, classification
equilibria as product favored or reactant favored, calculation of equilibrium concentrations using K and
Kp and ICE method, reaction quotient Q to predict which direction equilibrium is approached.
c) Recognize, define and apply Le Chatelier's principle to predict changes in concentrations, pressure or
volume, and temperature shift in chemical equilibria.
d) Recognize, define and apply equilibrium concept to industrial and biological catalysts.
Chapter 15
a) Recognize, define and apply the concepts acids/base in water, Arrhenius, Bronstead, Lewis acids/base
definitions, acid/conjugate base pairs in dissociation equilibria in aqueous solutions.
b) Recognize define various types of acids and bases: binary acids, oxy acids, organic acid, amines etc.
c) Recognize, define and apply the concepts dissociation equilibrium, Ka, Kb, % ionization, Kw, pKa, pKb,
pH, pOH etc.
d) Recognize the difference in approach in calculating pH and pOH of strong acid/base and weak
acid/base solutions.
e) Recognize and apply acid/base equilibria concepts to calculate Ka, Kb, Kw, pKa, pKb, pH, and pOH of a
given acid base solution.
Chapter 16
a) Recognize, define and apply the concept of buffers, Le Chatelier's principle and the common ion effect,
how they maintaining pH, calculate their pH using Henderson-Hasselbalch equation.
b) Recognize, define and apply the concept of solubility product Ksp of a slightly soluble salt.
Chapter 17
a) Recognize, define and apply the concept of Zeroth, first, second and third law of thermodynamics.
b) Recognize, define and apply the concept of enthalpy H (at constant temperature), internal energy U
(at constant volume), entropy S, G and G=H -TS.
c) Recognize, define and apply calculations of the entropy H change or free energy G for a chemical
reaction, from Hf or Gf.
d) Recognize, define and apply calculations of the entropy H change or entropy S change for a chemical
reaction, given series of thermochemical reactions and using Hesse’s law.
e) Recognize, define and apply connection between enthalpy G(standard), G (non-standard) and G =
G- RTln Q, for equilibrium reactions i.e. G= - RTln K, and G = - nFEcell to calculate G for
various chemical reactions.
Brief list of topics covered
Materials covered:
Chapter
Title
13.
Chemical Kinetics
14.
Chemical Equilibrium
15.
Acids and Bases
16.
Aqueous Ionic Equilibrium
17.
Free Energy and Thermodynamics
Sections
12.2, 12.3, 21.4, 12.5, 12.6, 12.7
13.2, 13.3, 13.4, 13.5, 13.6, 13.7
14.2, 14.3, 14.4, 14.5, 14.6, 14.7, 14.8 14.9
16.2, 16.3, 16.4
17.2, 17.3, 17.4, 17.5, 17.6, 17.7, 17.8, 17.9