Chemical Carbohydrate Model Lab
advertisement
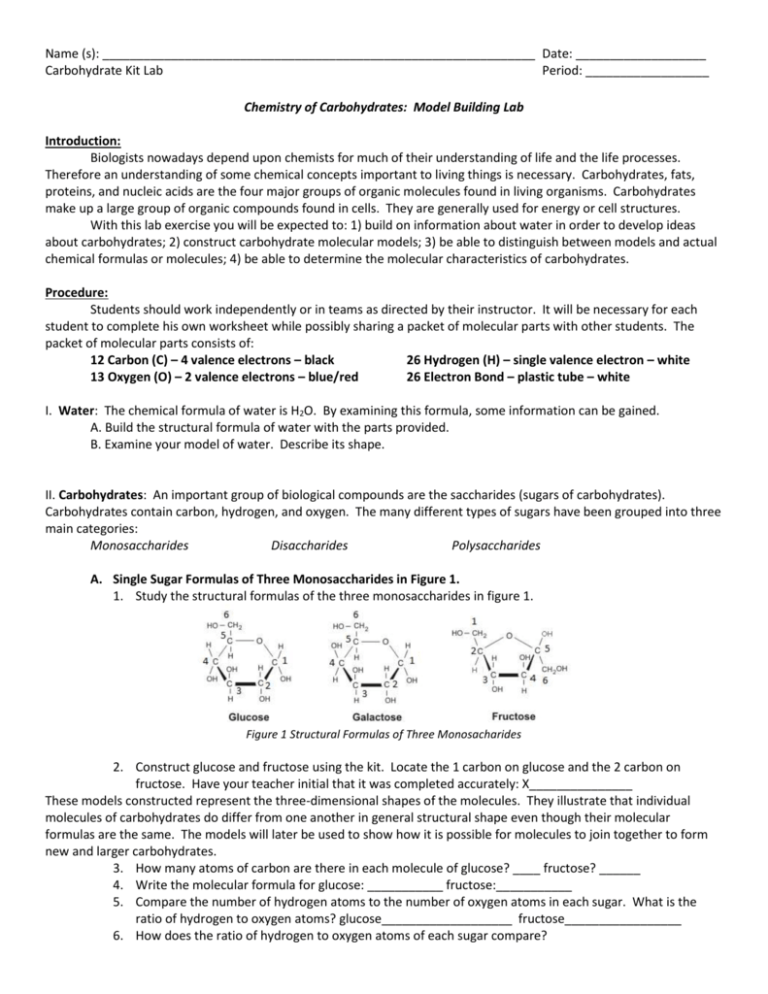
Name (s): _______________________________________________________________ Date: ___________________ Carbohydrate Kit Lab Period: __________________ Chemistry of Carbohydrates: Model Building Lab Introduction: Biologists nowadays depend upon chemists for much of their understanding of life and the life processes. Therefore an understanding of some chemical concepts important to living things is necessary. Carbohydrates, fats, proteins, and nucleic acids are the four major groups of organic molecules found in living organisms. Carbohydrates make up a large group of organic compounds found in cells. They are generally used for energy or cell structures. With this lab exercise you will be expected to: 1) build on information about water in order to develop ideas about carbohydrates; 2) construct carbohydrate molecular models; 3) be able to distinguish between models and actual chemical formulas or molecules; 4) be able to determine the molecular characteristics of carbohydrates. Procedure: Students should work independently or in teams as directed by their instructor. It will be necessary for each student to complete his own worksheet while possibly sharing a packet of molecular parts with other students. The packet of molecular parts consists of: 12 Carbon (C) – 4 valence electrons – black 26 Hydrogen (H) – single valence electron – white 13 Oxygen (O) – 2 valence electrons – blue/red 26 Electron Bond – plastic tube – white I. Water: The chemical formula of water is H2O. By examining this formula, some information can be gained. A. Build the structural formula of water with the parts provided. B. Examine your model of water. Describe its shape. II. Carbohydrates: An important group of biological compounds are the saccharides (sugars of carbohydrates). Carbohydrates contain carbon, hydrogen, and oxygen. The many different types of sugars have been grouped into three main categories: Monosaccharides Disaccharides Polysaccharides A. Single Sugar Formulas of Three Monosaccharides in Figure 1. 1. Study the structural formulas of the three monosaccharides in figure 1. Figure 1 Structural Formulas of Three Monosacharides 2. Construct glucose and fructose using the kit. Locate the 1 carbon on glucose and the 2 carbon on fructose. Have your teacher initial that it was completed accurately: X_______________ These models constructed represent the three-dimensional shapes of the molecules. They illustrate that individual molecules of carbohydrates do differ from one another in general structural shape even though their molecular formulas are the same. The models will later be used to show how it is possible for molecules to join together to form new and larger carbohydrates. 3. How many atoms of carbon are there in each molecule of glucose? ____ fructose? ______ 4. Write the molecular formula for glucose: ___________ fructose:___________ 5. Compare the number of hydrogen atoms to the number of oxygen atoms in each sugar. What is the ratio of hydrogen to oxygen atoms? glucose___________________ fructose_________________ 6. How does the ratio of hydrogen to oxygen atoms of each sugar compare? 7. How does the sugar ratio compare to the ratio found in water? _________________ 8. The structural arrangement of C, H, and O in glucose, fructose, and galactose differs. This helps explain why different shapes are used for each monosaccharide. Study the models of monosaccharides in Figure 1, these monosaccharides are considered isomers of each other. Look at their similarities and difference and describe what you think is an isomer. B. Double Sugars or Disaccharides: Two monosaccharide molecules can chemically join together to form a large carbohydrate molecule called a double sugar or disaccharide. When glucose joins with fructose, a different disaccharide is produced called sucrose. Is this process a chemical reaction? Yes or No 1. Pick up the glucose and a fructose model that was made in part A. Since each atom has the correct number of bonds, these compounds cannot be combined. Bonds must be broken to make products. 2. It will be necessary to remove an –OH on the number 1 carbon on glucose and an –H on the number 2 carbon on fructose in order to join the two molecules together. Remove –OH and –H ends and join these atoms together to form water. Take the open ends of the glucose and fructose models and join them together. Once they are joined, you have formed sucrose. Why is sucrose considered a double sugar? 3. Using your model kit. Try constructing maltose. Which two models will you need? ______________ and _________________________. Follow this rule: Remember: Remove the –OH from the #1 Carbon of one model and the and the –H from #4 carbon of the other model. 4. Write the molecular formula for maltose: __________________________ 5. Write the molecular formula for sucrose: __________________________ 6. What are the ratios of hydrogen atoms to oxygen atoms for both molecules? _____________________ 7. How does the ratio of hydrogen to oxygen compare in sucrose and maltose?____________________ in glucose and fructose? ____________________ in water? ___________________ 8. Does isomerization exist in disaccharides? Yes or No C. Complex Sugars or Polysaccharides: Just as disaccharides were formed from two monosaccharides, complex sugars are formed from combining single sugars chemically. The exact number of glucose molecules attached to form these polysaccharides is not known. The two most common polysaccharides are starch and cellulose. They consist of long chains of glucose molecules joined together. 1. With another team, construct starch by joining four glucose molecules. This represents only a small part of a starch molecule because starch consists of hundreds of glucose molecules. It will be necessary to dismantle previous made molecules in order to create the starch. 2. What must be removed from some glucose molecules in order to join them? ____________________ 3. Using only the middle “glucose” molecules of the model, determine the molecular formula for starch. (Remember- that only a molecule of water has been lost from each glucose molecule when it joined the others.) 4. How does the ratio of H to O atoms in starch with the ratio in disaccharides? _____________________ In single sugars? ______________ in water? _____________________ Interpretations: 1. Synthesis means the “process by which single compounds are united to form more complex materials”. Dehydration means the “loss of water”. Explain why chemists refer to the joining of monosaccharides to form polysaccharides as a dehydration synthesis reaction. 2. Why is the joining of four glucose molecules in forming a polysaccharide and example of dehydration synthesis? 3. The word carbohydrate is derived from carbon and water (hydrate). Explain why this combination correctly describes this chemical group.











