Hydrogen+ Carbon + Nitrogen = Hydrogen Cyanide (HCN
advertisement

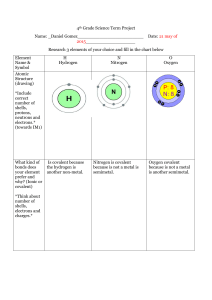
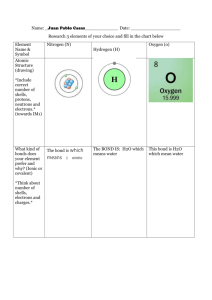
Hydrogen+ Carbon + Nitrogen = Hydrogen Cyanide (HCN) Harmless Elements: Hydrogen, Carbon, Nitrogen. Harmful Compound: Hydrogen Cyanide Chemical Bonding: Covalent Bonding Hydrogen (H): Hydrogen is the simplest element of all, and is the most common element in the entire Universe. Hydrogen is made up of 1 proton and electron, and no neutrons. It is a colorless and odorless gas which exists at standard temperature and pressure, as diatomic molecules, H2. Carbon (C): Carbon can exist in several allotropes, including graphite, diamond, amorphous carbon, fullerines and nanotubes. Carbon has the highest melting/sublimation point of all the elements, and is used as fuel. Nitrogen (N): Nitrogen is a colorless, odorless, tasteless and diatomic gas at standard temperature and pressure. It is used to produce fertilizers, and in current food production methods. Hydrogen Cyanide (HCN): Hydrogen Cyanide is a colourless, very poisonous, and highly volatile liquid that boils slightly above room temperature. The gas itself can explosive in air, at concentrations above 5.6 percent. Given its toxicity, hydrogen cyanide is listed as a warfare agent. (So that soldiers are not interrogated) Dot and Cross Diagram: X= Carbon Electrons = Hydrogen Electrons X Carbon Hydrogen X X XXX Nitrogen = Nitrogen Electrons Explanation: 1. The hydrogen has 1 valence electron, carbon has 4 nitrogen has 3. 2. The molecule is arranged exactly as it is spelled: H-C-N. 3. Carbon will give share electrons with both Nitrogen and Hydrogen. 4. Hydrogen needs 1 electron to complete a shell, so carbon shares 1 with hydrogen. Thus 2 electrons are being shared between them and carbon now needs 3 electrons to complete the shell. 5. Both Carbon and Nitrogen has needs 3 electrons to complete the shell, thus both elements share 3 electrons. Now, all 3 elements have full shells. (Hydrogen 2, Carbon 8, Nitrogen 8). 6. So, Between Hydrogen and Carbon- Single covalent Bond Between Carbon and Nitrogen- Triple covalent bond Done By: Alex Peng (2P4)