Covalent bonds week 5 homework
advertisement

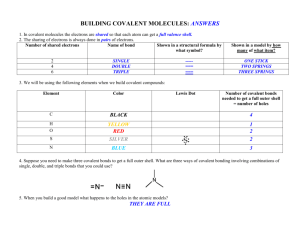
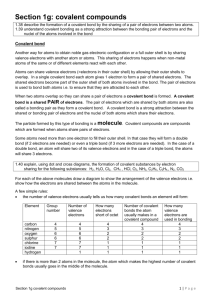
Covalent bonds week 5 homework 1. Identify what kinds of elements combine to form covalent compounds. None metal atoms share electrons to form covalent bonds. 2. Define the term ‘covalent bond’. A covalent bond is when a chemical is formed by the sharing of electrons. 3. Describe what an element’s electron dot diagram represents. An element’s dot diagram shows us how elements share electrons form covalent bonds. 4. Recall what properties most covalent compounds have in common. They usually exist as gases, liquids or solids and usually have a low melting point. They generally don’t conduct electricity. 5. Distinguish between a single covalent and a triple covalent bond, in terms of the number of electrons involved. 8. Explain why the noble gases don’t form covalent compounds. Noble gases don’t form covalent bonds because they have a complete structure so they don’t need to gain or give away any electrons. 9. Explain why CO2 (a compound) and O2 (an element) are both molecules. They are molecules because they are two atoms put together in one structure.