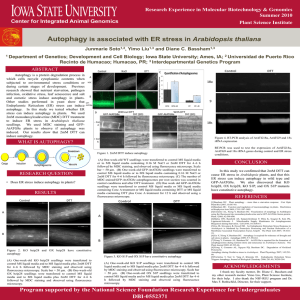
1. Autophagy - Utrecht University Repository
advertisement

By Elise Hovingh University of Utrecht The front cover depicts the artistic presentation of the four different viruses which will be covered in this thesis. Additionally the process of autophagy is depicted where the double membrane vesicle is derived from the ER and eventually fuses with the endosomes/lysosome in order to degrade its content consisting of organelles or other cellular/viral components. Images were taken from: http://www.turbosquid.com/3d-models/nucleus-cellendoplasmic-reticulum-max/609890 http://endosymbiotichypothesis.wordpress.com/evidenc e-for-the-endosymbiotic-hypothesis/ http://bilbo.bio.purdue.edu/~baker/projects/papova/pa pilloma/hpv_1L1L2.html http://www.sciencephoto.com/media/248957/view http://www.rkm.com.au/virus/coronavirus/coronavirusvirion.html http://www.newscientist.com/article/dn17049-expertanalysis-mexican-swine-flu--the-story-so-far.html Special thanks to my supervisor Dr. Fulvio Reggiori for a critical look at my manuscript and his guidance. 1|Page ABBREVIATIONS 4 ABSTRACT 7 1. AUTOPHAGY 8 1.1 INTRODUCTION 1.2 MECHANISM OF THE AUTOPHAGOSOMES FORMATION 1.3 REGULATION 1.3.1 MTOR 1.3.2 BECLIN 1 1.3.3 UNFOLDED PROTEIN RESPONSE 1.4 ANTIGEN PRESENTATION AND AUTOPHAGY 8 8 9 9 11 11 11 2 HERPESVIRUSES 13 2.1 INTRODUCTION 2.1.1 THE DISEASES 2.1.1.1 Alphaherpesviruses 2.1.1.2 Betaherpesviruses 2.1.1.3 Gammaherpesviruses 2.1.2 INFECTION MECHANISM 2.2 ALPHAHERPESVIRUSES AND AUTOPHAGY 2.2.1 HSV-1 AND AUTOPHAGY: ICP34.5 AND US11 2.2.1.1 HSV-1 and Alzheimer’s disease 2.2.2 VZV IS INCAPABLE OF BLOCKING AUTOPHAGY 2.3 BETAHERPESVIRUSES AND AUTOPHAGY 2.3.1 CMV AND TRF1 IN MODULATING AUTOPHAGY 2.3.2 MTORC MODULATION BY CMV 2.3.3 MOUSE CYTOMEGALOVIRUS (MCMV) AND M45 2.4 GAMMEHERPESVIRUSES 2.4.1 MSHV68 INHIBITION OF AUTOPHAGY THROUGH M11 2.4.2 V-FLIP, V-BCL-2 AND RTA OF KSHV 2.4.3 LMP1 AND EBNA1 OF EBV AND THEIR ROLE IN AUTOPHAGY 2.5 FUTURE DIRECTIONS 13 13 13 14 14 14 17 17 20 21 21 21 22 22 23 24 24 25 26 3 VACCINIA VIRUS 29 3.1 INTRODUCTION 3.1.1 DISEASE 3.1.2 INFECTION MECHANISM 3.1.3 ONCOLYTIC VIRUS 3.2 VACCINIA AND AUTOPHAGY 29 29 30 32 32 2|Page 3.2.1 REPLICATION 3.2.2 PKR PATHWAY 3.2.3 BCL-2 LIKE VIRAL PROTEINS 3.2.4 ATG12-ATG3 CONJUGATE 3.3 FUTURE DIRECTIONS 33 33 34 34 36 4. CORONAVIRUSES 39 4.1. INTRODUCTION 4.1.1 DISEASE 4.1.2 INFECTION MECHANISM 4.2 CORONAVIRUS AND AUTOPHAGY 4.2.1 HIGHJACKING LC3-I 4.2.1.1 The ERAD tuning pathway 4.2.1.2 DMVs and EDEMosomes 4.2.1.3 MODELS 4.2.2 INDUCTION OF AUTOPHAGY 4.3 FUTURE DIRECTIONS 39 40 40 42 43 43 43 44 45 46 5. INFLUENZA 49 5.1 INTRODUCTION 5.1.2 DISEASE 5.1.3 INFECTION MECHANISM 5.2 INFLUENZA AND AUTOPHAGY 5.2.1 INDUCTION OF AUTOPHAGY 5.2.2 REPLICATION 4.2.3 TO BLOCK OR NOT TO BLOCK 5.2.4 H5N1-INDUCED AUTOPHAGY-MEDIATED CELL DEATH 5.3 FUTURE DIRECTIONS 49 50 50 52 53 53 54 55 55 6. CONCLUSIONS 59 REFERENCES 62 3|Page Abbreviations ACE2 Angiotensin Converting Enzyme 2 AD Alzheimer Disease Atg Autophagy Associated proteins BBD Beclin Binding Domain CEV Cell associated Enveloped Virus CMV Cytomegalovirus CNS Central Nervous System CoV Coronavirus DC Dendritic Cell DFCP Double FYVE-Containing Protein DMVs Double Membrane Vesicle EBV Epstein-Barr Virus EEV Extracellular Enveloped Virus EGFR Epithelial Growth Factor Receptor EM Electron Micropscopy ERAD ER Associated degradation ERGIC ER-Golgi Intermediated Compartment EV Extracellular Virus GAGs Glycosaminglycans gB Glycoprotein B gC Glycoprotein C gH Glycoprotein H gL Glycoprotein L HA Hemagglutinin HSV Herpes Simplex Virus IBV Infection Bronchitis Virus ICP34.5 Infected Cell Protein 34.5 IMV Intracellular Mature Virus 4|Page IRE1 Inositol Requiring Enzyme 1 KHSV Kaposi Sarcoma associated Herpes Virus MCMV Mouse Cytomegalovirus MHC Major Histocompatibility Complex MHV Mouse Hepatitis Virus mTOR Mammalian Target Of Rapamcyn MV Mature Virus NA Neuroaminidase nAbs Neutralizing Antibodies NK Natural Killer NLS Nuclear Localisation Signal NP Nucleoprotein OIS Oncogene Induced Senesence PBIF2 Polymerase Basic Protein PDGFR Platelet Derived Growth Factor Receptor PE Phosphatidylethanolamine PERK Protein Kinase RNA-like ER Kinase PIP3K Phosphatidylinositol 3 phosphate-OH kinase complex PKR Protein Kinase R PtdIns(3)P Phosphatidylinositol 3 phosphate ROS Reactive Oxygen Species RTA Transcription Activator RVN Reticulovesicular Network SA Sialic Acid SARS Severe Acute Respiratory Syndrome TLR Toll Like Receptor TSC1/2 Tuberous Sclerosis Complex 1/2 UPR Unfolded Protein Response UTR UntranslatedRegion 5|Page v-FLIP viral FLICE protein vRNP viral Ribonucleoproteins VZV Varicela Zoster Virus WT Wild Type 6|Page Abstract Autophagy is an evolutionary conserved process for the recycling of cellular contents through the delivery of the targeted structures to the lysosome for degradation. Research on autophagy started in the late 1950s but only within the last two decades, the interaction between viruses and autophagy has become a topic of interest. Autophagy was shown to play a key role during the immune response against group A Streptococcus bacteria and the parasite Toxoplasma by preventing their replication. Additionally sinbis virus infection is compromised by autophagy. Conversely, autophagy was also proven to be pro-viral stimulating viral yields of both polio and dengue virus, amongst others. These observations have indicated that specific viruses can manipulate autophagy either positively or negatively in order to enhance their own replication. In this thesis the interaction between autophagy and four different viruses, i.e. herpes, vaccinia corona and influenza virus has been discussed. Herpesviruses can be divided into three categories: alpha, beta and gamma, and each class of herpesvirus display some similar characteristics regarding the interplay with autophagy. Differences are, however, also present. These differences cannot only be found between classes, but also within classes as well as between infections in different cell types. Interaction of vaccinia viruses and coronaviruses are less well defined compared to those of the herpesviruses. Although evidence exists for autophagy manipulation by vaccinia viruses, this has not been clarified for coronaviruses. Nonetheless within the coronavirus life cycle an autophagy protein but not an intact autophagy machinery, was shown to be involved in the replication of the virus suggesting that autophagy proteins may be involved in other cellular processes as well. Studies on the influenza virus have generated some conflicting data where a block in autophagy was shown by some research groups while others revealed the contrary. Discrepancies are, nevertheless, not only present in literature about the influenza virus but are also seen in that of herpesviruses. This could be due to the virus behaving differently when infecting different cell types. Current knowledge on the interaction between autophagy and these different viruses will be reviewed in this thesis along with the discussion of the discrepancies and models suggesting possible future lines of research. 7|Page 1. Autophagy 1.1 Introduction Autophagy is an evolutionary conserved process for recycling cellular contents by the delivery of targeted structures to the lysosome for degradation. The process requires a series of autophagyassociated genes (Atg), which generate double membrane vesicles (DMVs) called autophagosomes that sequester cytoplasm as well as cytoplasmic organelles. Autophagosomes have a diameter of about 0.5-1.5 µm and can subsequently fuse with multivesicular bodies, early endosomes or late endosomes forming an amphisome. Amphisomes then fuse with lysosomes, forming an autolysosome, in order to degrade its contents (Figure 1 A) (Crotzer et al. 2010). There are three different types of autophagy: Microautophagy, chaperone mediated autophagy and macroautophagy (Pyo et al. 2012). The latter will be discussed in this thesis and be referred to as autophagy. Autophagy is important in numerous physiological processes such as development, tumor suppression, prevention of neurodegeneration, T cell homeostasis and survival during starvation or growth factor withdrawal. Most importantly for this thesis, autophagy has also been shown to be important in different aspects of the immune system. It can engulf and degrade pathogens limiting their intracellular proliferation. Furthermore it can present antigens on the major histocompatibility complex (MHC) recruiting the adaptive immune response. Lastly, it can promote a pro-inflammatory response by presenting engulfed viral components to endosomal toll like receptors (TLR). The interaction between autophagy and some pathogens has been experimentally proven. For example, autophagy prevents group A Streptococci from replicating in the cytosol and targets intracellular parasite Toxoplasma (Nakagawa et al. 2004; Andrade et al. 2006). Infection with Sinbis virus has additionally been shown to be compromised by autophagy (Orvedahl et al. 2010). Autophagy can however also be pro-viral because its stimulation increases, for example, the yields of polio and Dengue virus (Levine et al. 2011). 1.2 Mechanism of the autophagosomes formation Autophagosome biogenesis starts with phagophore, a small membranous cistern, formation. These phagophores are subsequently expanded and close generating the double membrane autophagosomes (Figure 1 A). This process is initiated by sequential interactions between mammalian target of rapamcyn (mTOR) and the ULK1 protein complex. This complex consists of ULK1, mATG13, FIP200 and Atg101. Under normal conditions mTORC is active and interacts with the UKL1 protein complex phosphorylating ULK1 and mAtg13, which inhibits the ability of the complex to 8|Page associate with a membrane hence inhibiting autophagy. Inhibition of mTOR, which happens for example upon starvation , leads to the activation of ULK1 and subsequent translocation of the ULK1 complex from the cytosol to the ER or closely related structures. The next step is nucleation where the ULK1 protein complex recruits the class III phosphatidylinositol 3 phosphate–OH kinase complex (PIP3K). This complex contains VSP34, VPS15, Beclin 1 and Atg14. The active complex results in the production of phosphatidylinositol-3-phosphate [PtdIns(3)P] to initiate the recruitment of autophagy proteins to the sites of phagophore formation and expansion. Beclin 1 is very important for the nucleation process. Without Beclin 1 or one of the Atg members autophagy cannot proceed. Beclin 1’s interaction with VPS34 stimulates VPS34’s catalytic activity leading to the local synthesis of PtdIns(3)P. The phagophore formation and expansion are achieved by generating two protein conjugates in an ubiquitin-conjugation-like manner. Atg12 is activated by the E1-like enzyme Atg7 and then conjugated to Atg5 by E2-like protein Atg10. This complex subsequently assembles with Atg16L and associates with the elongating phagophore. The second conjugate is phosphatidylethanolamine (PE)-lipidated LC3 also called LC3-II. This conjugate is produced by Atg7 and Atg3, the E1- and E2-like enzymes, respectively (Figure 1 B). The Beclin1/VPS34/p150 unit is involved in several stages of autophagy depending on Beclin 1’s interactions. Binding to Atg14, as mentioned, above leads to recruitment onto the autophagosomal membrane however when bound to UVRAG the complex becomes involved in maturation of autophagosomes. Simultaneously binding both UVRAG and RUBICON, in contrast, makes the complex inhibitory as it prevents maturation of the autophagosomes (Pyo et al. 2012; Shi et al. 2012). The lipids that generate the autophagosomal membrane can either originate from the ER or the mitochondrion, though there have also been reports that the Golgi, endosomes and the plasma membrane can also be the origin of the autophagosomal membrane. The ER has been proposed as it targets Atg14, which is required for autophagy by regulating VPS34. Furthermore activation of autophagy recruits the ER-resident double FYVE-containing protein (DFCP), which binds PtdIns(3)P suggesting that this is derived from ER lipids. These ER subdomains containing DFCP are called omegasomes and recruit Ulk1, Atg14, Atg5 and LC3 (Levine et al. 2011). Mitochondria have been suggested as membrane origin as during starvation Atg5, which is essential for LC3-II recruitment, locates to the outer membrane of the mitochondria (Hailey et al. 2010). 1.3 Regulation 1.3.1 mTOR There are several ways through which autophagy can be regulated. The most important and well known pathway involves mTOR, which not only regulates autophagy but also protein synthesis and the import of nutrients. Inhibition of mTOR leads to the activation of autophagy through its 9|Page Figure 1: Autophagosome formation. A. Synthesis of an autophagosome starts with the formation of a phagophore which is subsequently enlarged and finally encloses the surrounding cytoplasmic content. They could contain protein aggregates, viral particles or even damaged organelles. The autophagosomes then fuse with endosomes or multivesicular bodies and eventually fuse with the lysosome, where their contents are degraded. B. The autophagy process can be set in motion by the mTOR complex. Upon inhibition of this complex by for example starvation, phosphorylation of the ULK1 kinase by mTOR is prevented. This allows UlK1 to be activated and in turn it phosphorylates FIP200 and mAtg13, two proteins in complex with ULK1, which leads to recruitment of PIP3K. This is in a complex composed of VSP34, p150, Beclin 1 and Atg14 on the site where the autophagosome will be generated. The complex initiates the elongation of the phagophore by recruiting the autophagy proteins Atg5, Atg12 and Atg16L. This results in LC3-I lipidation and incorporation into the phagophore membrane. Adapted from: Pyo et al. 2012 and Orvedahl et al. 2008 downstream targets ULK1 and ULK2. These result in the activation of autophagy via the pathway described in section 1.2. Treating cells with rapamycin, a natural inhibitor of MTOR, can mimic this activation of autophagy via MTOR. Upstream mTOR is inhibited by the heterodimer of the tuberous sclerosis complex 1 and 2 (TSC1/2). The complex of these two components with mTOR and raptor receives signals from multiple signaling pathways such as the one centered around class I PI3K and Akt. Akt for example phosphorylates TSC2 catalyzing its dissociation from the TSC1/2 complex and thus raises the inhibition of mTOR with the consequence of inhibiting autophagy. Additionally, high levels of amino acids result in the stimulation of Ras-related small GTPases, which activate mTOR 10 | P a g e (Cavignac et al. 2010). Conversely when the AMP:ATP ratio increases, indicating a lack of energy, TSC2 is activated by the AMP activated protein kinase (AMPK), which subsequently inhibits mTOR and activates autophagy (Gwinn et al. 2008). AMPK can also phosphorylate raptor, which results in the inhibition of mTOR allowing AMPK to directly phosphorylate ULK1 mediating autophagy activation (Egan et al. 2011). 1.3.2 Beclin 1 As explained, a decrease in amino acid concentration in the cell activates autophagy. This also occurs as JNK1 phosphorylates Bcl-2. Bcl-2 has the ability to bind Beclin 1 and thus to repress autophagy (Pattingre et al. 2005). JNK1 phosphorylation of Bcl-2 interferes with the interaction between Beclin 1 and Bcl-2. Beclin 1 is thus more available for autophagosomes formation. The interaction between Beclin1 and Bcl-2 can also be disturbed by the DAP kinase, which is activated by death signals, and TRIF and Myd88 which are activated by pathogens and hypoxia, respectively. The last two achieve this via the TLRs (Cavignac et al. 2010). 1.3.3 Unfolded protein response Cell stress induces the eIFα2 kinase signaling pathway, which has additionally been shown to increase autophagy activity. The exact mechanism via which this happens has not been entirely unraveled. There are four different kinases which phosphorylate eIFα2: GCNZ, protein kinase R (PKR), protein kinase RNA-like ER kinase (PERK) and HR1, which are activated upon starvation, viral entry, ER stress and heme deprivation, respectively (Esclatine et al. 2009). PERK has been shown to upregulate Atg12 and might induce autophagy in this way. PERK is additionally involved in the unfolded protein response (UPR) (Lai et al. 2007). The UPR is activated upon ER stress and copes with alterations in protein folding. It reduces the load of unfolded proteins via several mechanisms such as enlarging the ER membrane and by ensuring selective synthesis of proteins involved in quality control and protein folding. When the ER stress is irreversible, this pathway will assure that the cell goes into apoptosis. It is known that upon ER stress autophagy is activated probably to degrade damaged ER as well as protein aggregates (Hetz, 2012). Hence the UPR most likely, either via PERK or additionally via other pathways, induces autophagy. 1.4 Antigen presentation and autophagy Autophagy plays a role in antigen presentation on MHC II. Traditionally MHC II displays epitopes from extra cellular proteins or pathogens, such as bacteria, and triggers a CD4+ T cell-mediated immune response (Dengjel et al. 2005). However, 10-30 % of the ligand repertoire, the ligandome, consists of peptides derived from either cytoplasmic or nuclear proteins. This can partially be explained by autophagy mediating the loading of MHC II as autophagosomes frequently fuse with the loading 11 | P a g e compartments of MHC II, the endosomes (Levine et al. 2011). Blocking autophagy decreases the amount of intracellular epitopes displayed on the cell surface but it does not completely abolish the loading on MHC II. This indicates that other mechanisms may also play a role in this presentation. Enhancing this T cell mediated immune surveillance could be important when the cells are under possible dangerous stress conditions. It has been shown that during starvation, when multiple autophagosomes are generated, the ligandome of MHC II is altered, which can lead to a defect in the positive and negative T cell selection in the thymus (Levine et al. 2011). Increased autophagy decreases the amount of active cathepsins, which are proteases within the endocytic compartments. Impairment of the activity of these cathepsins leads to a less efficient degradation in the lysosome. The fact that the proteins are turned over less efficiently might favor their presentation on MHC II as the remaining epitopes are larger (Dengjel et al. 2005). Some viruses are able to modulate this role of autophagy, a topic that will be discussed in the following chapters. In this thesis the interaction of two DNA viruses, Herpesviruses and vaccinia viruses, and two RNA viruses, Influenza viruses and coronaviruses, with autophagy will be discussed. While some viruses seem to initiate autophagy and benefit from this induction others have evolved anti-autophagy viral proteins. Overall, however, the viruses cannot simply be divided in stimulants or inhibitors of autophagy considering the fact that some viruses behave differently in different cell types. Tropism seems to, at times, determine the negative or positive influence of the virus on the autophagy mechanism. Additionally the post infection time point can also play a part in the manipulation of autophagy. Although research on autophagy started in the late 1950s the link between these viruses and this pathway has only recently been more elaborated (Yang et al. 2010). There are still many unsolved issues regarding the interaction between, for example, the four viruses discussed in this thesis and autophagy. These discrepancies and queries will along with the current evidence be covered in the coming chapters. 12 | P a g e 2 Herpesviruses 2.1 Introduction Herpesviruses are common viruses characterized by their double stranded DNA genome, which consists of approximately 124-230 kilo bases. The genome is packed within an icosahedral nucleocaspid covered with tegument and enclosed in a membrane envelope (Engleberg et al. 4th edition). The herpesvirus family consists of three different subfamilies classified based upon their host range, replication cycle and cell tropism: The alphaherpesviruses, the betaherpesviruses and the gammaherpesviruses (Connolly et al. 2011). Herpes simplex virus (HSV), cytomegalovirus (CMV) and Epstein-Barr virus (EBV), respectively, are classic examples of these three subfamilies. Additionally Kaposi sarcoma associated herpes virus (KHSV) and Varicella Zoster virus (VZV) are also a known gammaherpesvirus and alphaherpesvirus, respectively. These viruses, which share the characteristic of establishing a life-long infection through latency once they have successfully infected an individual, will be discussed in this chapter (Murray et al. 5th edition). 2.1.1 The diseases 2.1.1.1 Alphaherpesviruses HSV-1 was the first herpes virus to be discovered (Pei et al. 2011). There are two different types of HSV, HSV-1 and HSV-2. The difference lies in the prefered way of transmission and where the symptoms arise. HSV-1 is spread mainly via oral-oral or via oral-genital contact. HSV-2 is primarily spread via genital-genital contact and is characterized by genital warts (Engleberg et al. 4th edition). In this thesis only HSV-1 will be further discussed.HSV-1 can result in corneal infection, which can lead to blindness but is commonly less severe and phenotypically characterized by cold sores. It is additionally the most common cause of sporadic encephalitis (Orvedahl et al. 2007). This type of encephalitis has a high rate of mortality without treatment (70%), which can be reduced to 20-30% with treatment (Overdahl et al. 2008). VZV, which is the causative agent of the chickenpox, is spread either via the respiratory route or through direct contact with lesions. VZV can reoccur at a later point in life and is characterized by chickenpox like lesions which are called zoster. Two thirds of the world population has HSV-1 antibodies as one fifth of sexually active adults have HSV-2 antibodies. Although a substantial amount of individuals are thus infected with an alphaherpesvirus, most of these infections are asymptomatic. Only one out of three infected individuals will display symptomatic disease. These are often the individuals with a weaker immune system (Engleberg et al. 4th edition). 13 | P a g e 2.1.1.2 Betaherpesviruses CMV infection tends to occur during adulthood. It is spread through direct contact as the virus is excreted in body fluids such as the urine and the saliva. Approximately 50-60% of all adults are infected with CMV. As with all herpesviruses, not all infected individuals display symptomatic disease. Nonetheless the virus will persist for life (Engleberg et al. 4th edition). CMV does, however, pose a main problem both concerning morbidity and mortality in immunocompromised individuals such as AIDS patients. In these patients it can cause multi-organ disease. CMV additionally causes postoperative illnesses as the virus can be spread upon blood transfusion as well as after organ transplantation. Furthermore, CMV can pass the placental barrier and may lead to congenital birth defects in the first trimester of a pregnancy (Boehme et al. 2012). 2.1.1.3 Gammaherpesviruses 90% of the world’s population is infected with EBV via oral transfer though, as the other herpesviruses, this is largely asymptomatic (Saha et al. 2011). EBV is associated with lymphoproliferative diseases being the causative agent of infectious mononucleosis, which occurs mostly during adolescence and is also known as the kissing disease. This disease is characterized by pharyngitis, fatigue, a low grade fever and peripheral blood monocytosis (Engleberg et al. 4th edition). Additionally EBV can be contracted as a result of transplantation and hence can cause posttransplant lymphoproliferative illnesses due to the suppressed immune system of the patient (Saha et al. 2011). EBV can furthermore result in neoplastic diseases. It was discovered in 1964 as the first cancer-related virus in the Burkitt’s lymphoma (Epstein et al. 1964). Moreover it can also lead to the development of Hodgkins disease (Saha et al. 2011). KSHV like EBV can cause several human cancers. It is the etiological agent of the Kaposi sarcoma, a cancer often seen in AIDS patients, and hence the result of a compromised immune system (Wah Wen et al. 2010). 2.1.2 Infection mechanism Generally the lytic and latent life cycles of the three different subfamilies of the herpesviruses are similar. There are, however, some differences that allow for classification of the families. The main differences lie in the cellular tropism as the different viruses infect different cell types as well as in the mechanism of entry into the cell. Herpesviruses require relatively many proteins during the entry process compared to other enveloped viruses which often only have a few proteins which mediate both entry and fusion (Connolly et al. 2011). HIV for example only needs gp120 to mediate the entry and fusion of the virus (Sun et al. 2006).HSV can infect lymphocytes, epithelial cells, fibroblasts and neurons. Depending on the cell type, the infection mechanism varies. Sometimes endocytosis is necessary, the exact reason for this is however unknown (Campadeli-Fiume et al. 2012). Three conserved viral glycoproteins, i.e. glycoprotein B (gB) and a heterodimer consisting of glycoprotein H 14 | P a g e (gH) and glycoprotein L (gL) (also known as gH/gL), are necessary for fusion of the virions with all the cell types. Glycoprotein D (gD) is also crucial for fusion. Furthermore glycoprotein C (gC) is needed to tether the viral particle with the cell plasma membrane by binding specific surface receptors such as the heparin sulphate proteoglycan or the TNF receptor facilitating entry (Connolly et al. 2011). Likewise the three conserved proteins are important for EBV cell entry as well. The binding activity of glycoprotein 42 (gp42) to HLAII for fusion with B cells is additionally important for entry. Glycoprotein 350/220 (gp350/220) is the tethering protein for EBV and binds CD21. EBV can also infect epithelial cells, which do not express HLAII and CD21. In this case entry is mediated via integrins (Connolly et al. 2011). CMV has the broadest cell tropism as it can infect nearly every cell in the body. According there are many different receptors to which CMV can bind such as integrins like αvβ3, platelet derived growth factor receptor (PDGFR), the TNF receptor family and the epithelial growth factor receptors (EGFR) (Soroceanu et al. 2008; Haspot et al. 2012; Wang et al. 2005; Feire et al. 2004). Additionally CMV can enter a cell in three different ways: Endocytosis, direct fusion or macropinocytosis (Haspot et al. 2012). The conserved protein gB, which is present in all herpesviruses, is the ligand for PDGFR and EGFR. The latter has been shown to be crucial for entry in some cases, for example in monocytes (Chan et al. 2009). This relevance, however, has been controversial as in fibroblasts, epithelial and endothelial cell lines EGFR was not required for entry (Isaacson et al. 2007). It was thus speculated that perhaps integrin heterodimers were sufficient for entry by interacting with gB and gH in these cells lines (Lopper et al. 2004). Perhaps EGFR serves to determine cellular tropism (Ryckman et al. 2008). Once the virus has successfully entered the cell, the capsid is transported to the nucleus releasing its DNA through the nuclear pores. Identically to all DNA viruses, the viral genes are then transcribed and the viral genome is replicated. Viral genes are transcribed in several steps starting with the immediate early genes, which subsequently drive the transcription of the early genes resulting in proteins needed for the replication of the viral DNA (e.g. viral DNA-dependent polymerase). The early gene products stimulate the expression of the late genes. These late genes encode the proteins that are needed to assemble and compose the progeny virions. Subsequently these virions bud through the nuclear membranes and obtain the final envelope by budding into cytoplasmic vesicles derived from the Golgi apparatus. Finally the mature virions egress via exocytosis (Engleberg et al. 4th edition; Mingo et al. 2012) (Figure 2). 15 | P a g e All herpesviruses are post infection persistently present throughout the life of the host through a mechanism called latency. HSV and VZV are latently present in the peripheral ganglia. They enter nerve extremities that are proximal to the epidermis and are subsequently transported to the bodies. During nerve cell HSV latency, similarly to other herpesviruses, the viral genome circularizes within the nucleus with minimal transcription of viral genes. Reactivation occurs when the immune system is compromised for example upon a Figure 2: General lytic life cycle of the herpes viruses. The herpesvirus enters via interactions occurring at the plasma membrane betweeb viral glycoproteins and host membrane surface proteins, which are different in between the diverse subfamilies. Subsequently the virion is transported to the nucleus where three sets of genes are transcribed at different times during the life cycle (immediate early, early and late genes). Subsequently these gene products ensure replication of the viral DNA and packaging into a nucleocapsid. The progeny virions bud out of the nucleus through the nuclear membranes. The final envelope is obtained by budding into a cytoplasmic vesicle of Golgi origin after which the virions leave the host cell through exocytosis. Modified from: http://www.studyblue.com/notes/note/n/lecture-20animal-viruses/deck/1405769 systemic infection, emotional stress sunburn, or ageing. Especially VZV reactivation causing Zoster is unpleasant resulting in vesicular lesions along the dermatome of the reactive virus containing nerve substantial amount causing of a pain (Engleberg et al. 4th edition). CMV latency is very poorly understood. The virus persists in hematopoietic progenitor cells as well as in macrophages maintaining large subsets of CMV specific T cells. Persistence could perhaps be better explained as a chronic controlled infection. To this end, insufficient T cell control leads to reactivation of the virus (Goodrum et al. 2012). For EBV, as opposed to the other herpesvirus classes, latency is the default pathway (Speck et al. 2010). Several specific latency proteins such as EBNA-1-6 and LMP1/2 regulate a complex process determined by alternative splicing maintaining latency in the memory B cells. EBV persistence in latent infected memory B cells induces proliferation by encoding proteins that are essential for the B cell 16 | P a g e proliferation. Thus loss of immunological control of EBV could result in hematopoietic malignancies such as Burkitt’s and Hodgkin’s disease (Saha et al. 2011). 2.2 Alphaherpesviruses and autophagy 2.2.1 HSV-1 and autophagy: ICP34.5 and Us11 The link between autophagy and HSV-1 infection was first made in 1979 when, by using electron microscopy (EM), an HSV-1 virus was observed inside an autophagosome (Smit et al. 1979). The ability of HSV-1 to inhibit autophagy has been extensively studied. There seems to be a cell specific and perhaps even time specific modulation of autophagy by HSV-1 showing distinct regulation in permissive and non permissive cells. Nevertheless, considering the fact that neurons and dendritic cells (DCs) are both non permissive for the initial infection but display different autophagy responses towards, this classification might not be as straightforward (Ramussen et al. 2011). In fibroblasts and neurons HSV-1 makes use of the infected cell protein 34.5 (ICP34.5), encoded by the neurovirulence gene gamma134.5, to counteract autophagy. ICP34.5 does this in two distinct ways (Figure 3). It interferes with the double stranded RNA dependent PKR, which is normally activated by type 1 interferons and phosphorylates the translation initiation factor eIF2α. This phosphorylation mediates host cell shut down as eIF2α-P can no longer carry out its function of recognizing start codons. This pathway is also important for the induction of autophagy. This gives the virus two exquisite reasons to interact with this pathway: The host cell shut down promotes replication and the block of autophagy prevents virus degradation (Alexander et al. 2007). Interference with the PKR pathway is mediated via direct binding of PPIα to the GADD34 domain of Figure 3: ICP34.5 blocks autophagy. IPC34.5 can block autophagy through two mechanisms. It binds PPIα with its GADD34 domain activating counteracts the it, which function of in PKR turn by dephosphorylation of eIF2α. Furthermore it binds Beclin 1 with its BBD (depicted in black) sequestering this protein and consequently disabling Beclin 1 to functionally induce autophagy. Us11, which is expressed late during the infection, also plays a role in inhibiting autophagy. It blocks PKR by either binding directly or by interfering with PACT, an activator of PKR. Adapted from: Orvedahl et al. 2007 17 | P a g e ICP34.5. PPIα is subsequently activated and dephoshorylates eIF2α (Orvedahl et al. 2007). In vitro infection with the ICP34.5-/- virus resutling in the inability to prevent autophagy has shown that this pathway does not seem to be important for replication. Replication defects in vitro are caused by the host cell shut off. In vivo, in contrast, this inability to control autophagy is in fact important for replication perhaps due to the fact that autophagy is a potent anti viral mechanism giving the host cell the overhand in clearing the virus (Leib et al. 2009). Furthermore Us11 is also involved in blocking autophagy. Us11 is a double-stranded RNA binding protein expressed late during infection. It antagonizes the PKR pathway either by direct binding or by interacting with an activator of PKR, PACT (Peters et al. 2002). Early expression of this protein in an ICP34.5 knock out virus leads to inhibition of autophagy by allowing the virus to dephosphorylate eIF2α (Alexander et al. 2008). This is, however, not a physiological situation but does indicate that Us11 has the ability to interact with the PKR-mediated induction of autophagy and block it. This is backed up by the expression of Us11 in HeLa cells and fibroblasts which was shown to block autophagosomes formation and hence autophagy suggesting that Us11 is, apart from ICP34.5, also able to mediate autophagy (Lussignol et al. 2012). Additionally ICP34.5 also associates with Beclin 1. Beclin 1 is essential for the formation of autophagasomes through its interaction with VPS34 as mentioned in the introductory chapter (Cavignac et al. 2010). ICP34.5 binds Beclin 1 directly with its beclin 1-binding domain (BBD). The exact site where ICP34.5 binds to Beclin 1 is however unknown. Interfering with Beclin 1 results in inhibition of autophagy. Indeed HSV-1 viruses without the BDD are unable to block autophagosome formation and are cleared more efficiently even when the GADD34 domain is still present. Thus PKR inhibition without Beclin 1 inhibition is not sufficient to block autophagy (Orvedahl et al. 2007). Additionally there is a difference in efficacy of replication between the BDD -/- virus and the wild type (wt) virus showing a lower rate of replication by the BDD-/- virus. This difference is principally displayed at a late infection time point suggesting the BDD lacking viruses are not able to overcome the adaptive immune system. Upon infecting a mouse without functional B and T cells with the BDD /- virus or wt virus, this difference in replication is no longer seen (Leib et al. 2009), suggesting that the adaptive immune system is triggered when autophagy is not blocked. Endogenous viral antigens can be presented on MHC complexes in non-permissive cells such as macrophages and DCs, through autophagy (i.e. cross-presentation) (see 1.4). In macrophages autophagy is distinctly regulated depending on the phase of the infection. At the early time points ICP34.5 inhibits autophagy similarly to what observed in fibroblasts and neurons. Late in the infection, however, the macrophages display a novel form of autophagy. This form involves the formation of autophagosomes derived from the coiling process of the nuclear membrane. These 18 | P a g e Figure 4: Formation of a four-layer membrane autophagosome derived from the nucleus. Schematic representation of the formation of a fourlayer membrane autophagosome derived from the nucleus. 1) Viral capsids assemble in the nucleus. 2) HSV-1 capsids acquire their envelope in the perinuclear space by fusing with the nuclear inner membrane. 3) Fusion with the outer nuclear membrane releases a naked HSV-1 capsid in the cytoplasm. Upon translocation through the nuclear membranes and release into the cytoplasm, the capsids can become trapped by the emerging four-layer membrane autophagosomse. These can subsequently detach from the nucleus as shown by the arrow. Adapted from: English et al. 2009 autophagosomes as opposed to the classical 2 membrane autophagosomes contain a 4-layer membrane structure containing a large amount of the viral gB protein (Figure 4). This suggests that inhibition of the classical autophagy route triggers this new form of autophagy, which leads to the presentation of degraded viral peptides such as pG on MHC complexes, thus triggering an immune response (English et al. 2009a; English et al. 2009b). The exact molecular mechanism of this novel type of autophagosomes is not unraveled yet. DCs are antigen presenting cells that are also manipulated by HSV-1. Unlike in the previously mentioned cells, HSV-1 is not capable of inhibiting autophagy in these cells. Autophagy is induced independently of the phosphorylation status of eIF2α but dependent on the presence of the viral genome in the cytosol, which is detected by STING. STING is an important part of the cytosolic DNA sensor system that among others induces type 1 IFN responses. The exact sensors through which the viral DNA induces autophagy remain unknown. Autophagy inhibition leads to a decrease in IFN-β production unveiling a link between autophagy and the innate immune response. The exact interaction between IFN, STING and autophagy has, however, not been discovered. Up to date no 4-layer membrane autophagosomes have been found in DCs (Ramussen et al. 2011). Nonetheless EM was not used to truly determine presence of these structures. Considering the fact 19 | P a g e that viral DNA in the cytosol induces autophagy, which cannot be overruled by ICP34.5, this fact raises the question why this phenomenon does not happen in other (permissive) cells and whether it can perhaps be connected to the formation of the 4-layer membrane autophagosomes. There is one report in literature that shows autophagy induction at a very early stage of HSV-1 infection in fibroblasts. In this work the authors proposed that the reason for the upregulation of autophagy might be due to the presence of viral DNA in the cytoplasm, which can be sensed by DNA sensors such as STING which was mentioned before (McFarlane et al. 2011). This hypothesis is in line with the induction of autophagy in DCs. In fibroblasts, however, autophagy induction does not remain dominant as autophagy is repressed later during infection. HSV-1 does not block the biogenesis of autophagosome in DCs but rather prevents their maturation. This is illustrated by an accumulation of the autophagosome cargo protein p62, which is not degraded due to the decreased flux. This inhibition of autophagosome maturation depends on the BBD of ICP34.5 and interferes with the immune system as the viral peptides do not reach the membrane for presentation (Gobell et al. 2011). Hence though HSV-1 seems to stimulate an innate immune response it hampers the adaptive response necessary to clear the virus. 2.2.1.1 HSV-1 and Alzheimer’s disease HSV-1 and its ability to bypass autophagy through its inhibition has been speculated to play a role in the development of Alzheimer’s disease (AD). In the elderly HSV-1 DNA is present in large amounts in areas of the brain which are often affected in AD. This is not seen in younger people suggesting that HSV-1 only reaches the brain once the immune system has been slightly compromised due to ageing. Nonetheless nearly every individual is infected with HSV-1 though not everyone develops AD. Therefore there must be other host factors that determine the susceptibility to AD. Likewise not all individuals infected with HSV-1 develop cold sores additionally indicating that host susceptibility is not only dependent on infection but also on other factors. The ApoE protein is a known factor increasing susceptibility to AD. How HSV-1 and ApoE interact remains unknown. They could possibly interact at an immunological level where ApoE would make the infected individual less successful in fighting the infection or they could perhaps interact at the cell surface as they both bind to the same receptor, i.e. HSPG (Itzhaki et al. 2008). Be that as it may solely the fact that HSV-1 inhibits autophagy might already be very important in developing the β-amyloid plaques, which are characteristic for AD. As autophagy is crucial in clearing aberrant proteins, its inhibition would leave the amyloid proteins untouched allowing the formation of plaques (Orvedahi et al. 2008.). Nonetheless further research is necessary to pinpoint the exact role of HSV-1 and autophagy in the development of AD and other neurodegenerative or auto mmune diseases of the central nervous system (CNS). 20 | P a g e 2.2.2 VZV is incapable of blocking autophagy VZV has the smallest genome of the alphaherpesviruses and does not contain an ortholog of the HSV-1 γ134.5 gene or a homolog of viral Bcl-2 present in gammaherpesviruses (see below). It is thus unable to block autophagy (Cavignac et al. 2010). Instead it appears to stimulate autophagy in the late stages of infection, both primarily and during reactivation, which is seen in both melanoma cells and fibroblasts (Takahashi et al. 2009). A different study, however, showed that the induction of autophagy by VZV in fibroblast is already present early during infection. This induction of autophagy is the result of increased viral glycoprotein synthesis leading to ER stress. The protein XBPI was present in VZV infected fibroblasts providing proof that the increased ER stress indeed activates the UPR, which induces autophagy. XBPI is a protein that is expressed when inositol requiring enzyme 1 (IRE1) processes its specific mRNA. The IRE1 pathway is activated by the UPR. In the case of VZV infection, autophagy might be proviral considering the fact that it is already active at an early time point of the infection (Carpenter et al. 2011). The induction of autophagy would prolong the life of the infected cell by reducing apoptosis which would give rise to the beneficial consequence of allowing the virus to propagate. 2.3 Betaherpesviruses and autophagy 2.3.1 CMV and TRF1 in modulating autophagy The interplay between CMV and autophagy appears to be more complex than with the alphaherpesviruses (Fig. 5). CMV infection, like HSV-1, also induces autophagy at an early phase of infection (McFarlane et al. 2011). It has been proposed that this could be due to presence of viral DNA in the cytoplasm or perhaps the induction lies in the virus binding to HSGPs, which are necessary for the viral cell entry. Nonetheless 24 hours post infection the tide turns and autophagy is blocked. This block depends on de novo synthesis of viral proteins as UV inactivated virus is not able to inhibit autophagasome formation. CMV does not contain an ortholog for either γ134.5, viral Bcl-2 or v-FLIP (see below) (Chaumorcel et al. 2012). It does however have a homolog of ICP34.5 namely TSR1. TSR1 has been shown to bind PKR directly inhibiting its phosphorylation of eIf2α (Hakki et al. 2006). Thus like HSV-1, this results in inhibition of host shut off and perhaps it is also involved in a block of autophagy. The N-terminal domain of TSR1 can also bind Beclin 1. To determine whether both binding sites were crucial for autophagy inhibition, mutant version of TSR1 lacking the binding domains have been made. The mutant that did not contain the PKR binding site still managed to inhibit autophagy and, therefore, like in HSV-1, the interaction with Beclin 1 is essential. Surprisingly infection with a TSR1 defective mutant does not lead to a stimulation of autophagy although inhibition is lost. This might be due to the fact that CMV has another highly homologous protein, IRS1, which could be functionally redundant with TSR1. These proteins have identical amino termini 21 | P a g e suggesting that IRS1 should also be able to bind Beclin 1, be it probably less efficiently than TSR1 (Chaumorcel et al. 2012). 2.3.2 mTORC modulation by CMV Another path via which CMV modulates autophagy is by acting on the mTORC pathway. Activation of mTORC kinase activity leads to inhibition of autophagy. mTORC activation leads to, amongst others, phosphorylation of 4EBP1 and p70S6K. These two proteins are involved in protein synthesis. Cells infected with CMV show an increased phosphorylation of both proteins suggesting activation of mTORC. Infected cells are even resistant to rapamycin, an inhibitor of mTORC (Chaumorcel et al. 2008). CMV influences mTORC by for example regulating TSC1/2 through the viral protein Ul38. TSC1/2 is a negative regulator of mTORC which is activated by CMV’s Ul38 (Esclatine et al. 2009). In CMV infected cells even starvation conditions which normally inactivate mTORC are unable to counteract the action of CMV and thus mTORC remains active and perinulclear keeping autophagy inhibited (Clippinger et al. 2011). This indicates that the interaction of CMV with the mTORC pathway is very potent at inhibiting autophagy. 2.3.3 Mouse cytomegalovirus (MCMV) and M45 CMV inhibits autophagy 24 hours post infection but also seems to make use of autophagy to overcome the immune system. The viral protein M45 in MCMV inhibits activation of TLR2, TLR3, TLR4, TRL7, TLR9, TNFR and NFκB. M45 was previously known to bind RIP1, a protein downstream of TNFR, TLR3 and TLR4. This, however, does not explain the inhibition of TLR2, TLR7 and TLR9, which are not controlled by RIP1. The C-terminal RNR R1 homology domain of M45 has been shown to interact with NEMO. NEMO is the scaffold of the IKK complex downstream of RIP1 (Fliss et al. 2012). Upon activation of the IKK complex, NEMO degrades the inhibitor of NFκB resulting in the activation of NFκB. Activation of NFκB is thought to be necessary at the start of infection as it mediates the transcription of the immediate early genes (Israel, 2010). CMV infection results in NEMO degradation through the targeting of NEMO by M45 to the autophagosome (Fliss et al. 2012). By degrading NEMO the IKK complex is unable to activate hence NFκB will remain bound to its inhibitor. This block on NFκB already starts at an early time point post infection but is only fully established later during the infection (Montag et al. 2006). Considering the fact that autophagy is inhibited 24 hours post infection, the degradation of NEMO via autophagy will be less efficient from this time point onwards. This might be the reason why M45 targets both NEMO and RIP1 as once the NEMO route becomes less efficient, the virus can subvert to the RIP1 pathway, which is still intact. 22 | P a g e Figure 5: CMV interacts with autophagy via TRF1 and M45. CMV induces autophagy early in infection but inhibits autophagy 24 hours post infection. This is achieved by activating mTORC through for example Ul38 interacting with TSC1/2, a negative regulator of the mTORC complex, which inhibits autophagy as well as activates protein synthesis. mTORC activation can be determined by phosphorylation of 4EBP1 and p70S6K. Furthermore the viral protein TSR1 and its homologue IRS1 which are the homologues of HSV-1‘s ICP34.5 play a role. TRF1 binds PKR inhibiting phosphorylation of eIF2α. This could also inhibit autophagy even if the binding to Beclin 1 of TRF1 has been shown to be the critical interaction to block autophagy. MCMV also contains the protein M45 which can bind the scaffold of the IKK complex, NEMO, and target it to the autophagosome thus making use of autophagy. Degradation of NEMO results in the inability of the complex to degrade the inhibitor of NFκB and hence NFκB will not be able to function as a transcription factor. 2.4 Gammeherpesviruses Gammaherpesviruses promote or suppress autophagy depending on the virus, cell context and whether the virus is in the lytic or latent stage (Figure 6). EBV, KSHV and MHV68 will be discussed in this section. All these viruses express Bcl-2 orthologues. Bcl-2 has an anti apoptotic function in the cell but it has also been shown to be an inhibitor of autophagy by binding Beclin 1 indicating that these two pathways are closely regulated. V-Bcl-2 also known as orf16 and M11 in KSHV and MHV68, respectively, are both able to bind Beclin 1 and thus inhibit autophagy (Cavignac et al. 2010. V-Blc-2 binds Beclin 1 with its BH3 binding domain, which consists of a hydrophobic pocket (Sinha et al. 2008). The binding between Beclin 1 and M11 is much stronger than the binding of Bcl-2 to for example bax or bak, which are pro-apoptotic proteins. This bound complex is not easily displaced suggesting that is likely very potent in inhibiting autophagy (Ku et al. 2008). 23 | P a g e 2.4.1 MSHV68 inhibition of autophagy through M11 Two distinct domains of MHV68’s M11 perform its anti-apoptotic and the anti-autophagy roles. Beclin 1 is bound by the α1 helix of the hydrophobic groove while bax and bak are bound by the BH3 domain. The anti autophagy property of M11 was shown to be dispensable during the lytic stage but crucial during latency. The anti-apoptotic property, in contrast, is important during reactivation (Xiaofel et al. 2009). There are, however, contradicting studies where a M11 defective mutant had no effects on latency and another where there was some initial reduction in latency induction was found but that this resolved 6 months post infection (Lima et al. 2005; Ganappa et al. 2002). MHV68 inhibition of autophagy during the lytic stage has not been described yet though an anti apoptotic protein important in the lytic phase, vMAP, had been discovered (Xiaofel et al. 2009). Considering the fact that autophagy and apoptosis are both inhibited by M11, perhaps this protein is able to negatively regulate autophagy in the lytic phase. Conflicting with the idea that autophagy might be inhibited during the lytic phase, autophagy induction has been related to survival of detached epithelial cells infected with MHV68. Inhibition of autophagy in these neoplastic cells causes cell death (Suarez et al. 2011). In this case viral persistence was aided by inducing autophagy again hinting to a divergence in autophagy modulation depending on the cell type and context. The exact mechanism is still subject of ongoing research. 2.4.2 v-FLIP, v-Bcl-2 and RTA of KSHV KSHV’s v-Bcl-2 actively suppresses autophagy during lytic replication of the virus also by binding to Beclin 1 (Liang et al. 2008). Additionally KSHV encodes the viral FLICE inhibitory protein (v-FLIP), which inhibits autophagy by binding Atg3 and thus preventing autophagosome elongation via inhibition of LC3 lipidation. V-FLIP is important in the inhibition of oncogene-induced senescence (OIS). OIS is the result of v-cyclin. This viral protein leads to the activation of a DNA damage-sensing pathway followed by mTOR inhibition, which leads to autophagy activation. Autophagy is in this sense the Achilles heel of the cellular senescence response targeted by v-FLIP (Leidal et al. 2012). Preventing the infected cell to stop proliferating is important for viral persistence and explains why KSHV can cause cancer. The v-FLIP has also been shown to protect epithelial cells from anoikis and thus contributes to the oncogenic characteristics of KSHV. KSHV is therefore able to target autophagy at two different phases, v-Bcl-2 which completely stops the formation of autophagosomes and v-FLIP which counteracts autophagosome elongation. The previously mentioned M11 protein lies in the genomic locus that v-FLIP is expected to occupy in MSHV68. Hence perhaps M11 does not only inhibit autophagy by binding Beclin 1 but also acquires the ability to function as v-FLIP by blocking the hosts’ antiproliferative response (Leidal et al. 2012). 24 | P a g e On the other hand KSHV has also been shown to induce autophagy during its lytic reactivation via the transcription activator (RTA). RTA, which is present in the nucleus, indirectly degrades the repressors of lytic reactivation by modulating the ubiquitin-proteasome pathway and autophagy. The mechanisms employed by RTA for the modulation of autophagy are unknown. It could potentially be due to an induction of the expression of autophagy-related proteins. This degradation process, however, needs to be tightly regulated limiting the replication in order to bring the virus back to its default latent stage since (Wen et al. 2010). To this end the v-Bcl-2 could bring the RTA-driven lytic reactivation back to latency by inhibiting autophagy. This nicely portrays the different requirements for autophagy in different phases of the infection cycle. 2.4.3 LMP1 and EBNA1 of EBV and their role in autophagy The proteins BALF1 and BHRF1 are two v-Bcl-2 proteins produced by EBV. Both are expressed early during the lytic cycle and the latter is also present during latency. It is, however, not known whether these proteins are able to bind Beclin 1 and whether they have an effect on autophagy (Taylor et al. 2011). Nonetheless, it has been shown that LMP1 regulates autophagy during latency. The amount of LMP1 expression has an effect on the level of autophagy induction. LMP1 is a transmembrane protein and is one of the 9 EBV proteins still transcribed during latency. LMP1 regulates its own synthesis and degradation via interplay with the UPR and autophagy. An intermediate level of LMP1 is needed to drive efficient B cell proliferation which is beneficial for the virus (Lee et al, 2008). LMP1 is an ambiguous molecule as it stimulates UPR and autophagy eventually inducing apoptosis through its 6 transmembrane domain so that it can regulate its own levels. It subsequently blocks apoptosis by signaling via the C-terminus. Apoptosis is inhibited by inducing expression of the cellular Bcl-2 homolog, Bcl-2a1. Cellular Bcl-2 has been shown to be able to bind Beclin 1 so perhaps the homolog could also inhibit autophagy when LMP1 should no longer be degraded. Furthermore the two v-Bcl-2 proteins might also play a part if they indeed have the ability to inhibit autophagy. This overexpression of the cellular Bcl-2 homolog, however, does not seem to fully inhibit autophagy. The exact manner how LMP1 triggers autophagy, directly or indirectly, is unclear (Pratt et al. 2012). Another latency protein, EBNA1, also interacts with autophagy. In this case, however, the interaction with autophagy does not appear to be beneficial for the virus. EBNA1 is presented on MHCII via autophagy stimulating a response mediated by CD4+ T cells. Normally EBNA1 localizes to the nucleus, which partially protects it from being displayed on the cell surface. Indeed when EBNA1 is artificially modified to be cytoplasm-bound, the amount of EBNA1 epitopes on MHCII significantly increases. 25 | P a g e Figure 6: Gammaherpesviruses interact with autophagy in divergent ways. Beclin 1 is a protein necessary for autophagosome formation. Viral Bcl-2 proteins, MSV68’s M11 and the v-Bcl-2 of KSHV, bind to Beclin via their hydrophobic groove and inhibit autophagosome formation. KSHV additionally contains another protein that inhibits autophagy during latency, i.e. v-FLIP. This protein associates with Atg3, an important component of the autophagy machinery, and inhibits autophagy. During lytic reactivation, however, KSHV promotes autophagy indirectly via RTA. EBV also has two v-Bcl-2 proteins but these have not been proven to bind Beclin 1. EBV does induce autophagy via a yet undetermined mechanism triggered by the latent membrane protein LMP1. Question marks indicate that the exact mechanism is still unknown. 2.5 Future Directions It is clear that autophagy plays an important role during the latent and lytic phases of the life cycle of the herpesviruses. Not all herpesviruses interact with autophagy through the same mechanisms and for the same reasons. Some viruses even seem to have developed different subversion strategies in different types of cells. The most important protein that herpesviruses exploit to inhibit autophagy is Beclin 1 as all of the viruses discussed, with the exception of VZV, have developed a way to interact with this protein. This protein will come back in the coming chapters as well as it seems to be a protein which is universally targeted by viruses. HSV-1 and CMV achieve this through the ICP34.5 and its homolog TSR1 respectively. The gammaherpesviruses do this with their v-Bcl-2 proteins, with a remaining question mark concerning the v-Bcl-2’s of EBV (Chaumorcel et al. 2012; Cavignac et al. 2010). For the v-Bcl-2 of MSHV68, M11, contradicting results have been obtained regarding its importance during latency (Lima et al. 2005; Ganappa et al. 2002; Xiaofel et al. 2009). This is due to differences in the design and execution of the studies as different doses and administration routes were used. In order to obtain a solid conclusion concerning the role of M11, additional investigations will need to be carried out under the same experimental conditions. The fact that during TSR1 deficient CMV 26 | P a g e infection autophagy is neither inhibited nor further induced was suggested to be due to the presence of the homolog IRS1 (Chaumorcel et al. 2012). To prove this redundancy, it might be interesting to look at the impact on CMV’s life cycle and autophagy when removing both proteins, as well as whether production of IRS1 increases to compensate when TRF1 is no longer present. Additionally it has not been shown whether IRS1 truly binds Beclin 1 hence a co-immunoprecipitation assay could be used to determine whether IRS1 and Beclin 1 are binding partners. In macrophages HSV-1’s negative regulation of autophagy results in the induction of a different kind of autophagy that gives rise to 4-layer nucleus-derived autophagosomes (English et al. 2009a; English et al. 2009b). Looking at whether this is a cellular response to this block or whether this is induced by the virus itself would be a very interesting next step in the investigation of these unique autophagosomes. Here one could for example look at whether gene expression is different between the early and late infection phases or whether deleting parts of the virus proteins will abolish the formation of these structures. Autophagy is activated by a few herpesviruses as well. VZV seems to induce it to keep the host cell alive and permits maintenance of the optimal conditions for replication (Takahashi et al. 2009). If this is indeed the case for VZV, these data somehow contradict data obtained for HSV-1, which show that this virus greatly benefits from inhibiting autophagy to block viral degradation and antigen presentation at the plasma membrane. Accordingly, activation of autophagy in HSV-1-infected cells leads to a decrease of viral potency. It might be interesting to look at the effect of inhibiting autophagy in cells infected with VZV, which should reduce VZV fitness. KSHV also stimulates autophagy through RTA upon reactivation of the virus. The exact mechanism how RTA induces autophagy remains speculative but might be found by analyzing gene expression pre and post RTA activation of autophagy. EBNA1, protein involved in EBV latency, is presented by MHCII via autophagy though as it resides in the nucleus, it is not entirely clear how it is exposed at the plasma membrane. Perhaps EBV also triggers the formation the 4-layer nuclear membrane derived autophagosomes, which could explain why nuclear antigens are transported on the cells surface through MHCII. This is something interesting to be investigated. Although the process is probably not very efficient because the antigens are only displayed in small quantities, the mechanism of antigen presentation would be interesting for further investigation. Additionally EBV encodes for two v-Bcl-2 proteins of which it is not known whether they bind Beclin 1 (Taylor et al. 2011) to inhibit autophagy for example during the lytic phase as activation of this pathway seems to be important during latency. Whether the v-Bcl-2 proteins of EBV are able to bind Beclin 1 will need to be further studied by coimmunoprecipitation for example. The fact that like KSHV, EBV can cause lymphoproliferative disease and even cancer suggests that EBV will also need to overcome OIS (Leidal et al. 2012). To this end EBV might contain a functional counterpart of the v27 | P a g e FLIP protein. However it seems that intermediate LMP1 levels cause proliferation of B cells during EBV infection so could also lead to lymphoproliferation. Perhaps there is a v-FLIP like protein that aids LMP1 in regulating autophagy when autophagy activity needs to be decreased thus inhibited. In conclusion, while herpesvirus infection progression has extensively been linked to autophagy there are many questions that will need to be addressed by further research; starting from what determines the different outcomes by the same virus in different cells to whether we can extend the information gained on one virus to a sub- or even an entire virus family. The route to full understanding of herpesvirus-autophagy interaction is nowhere near the end. 28 | P a g e 3 Vaccinia virus 3.1 Introduction Vaccinia virus is the virus that was used in the smallpox vaccine which lead to the eradication of smallpox in 1977. It belongs to the same genus, Orthopoxviruses, as the variola pox virus, which is the causative agent of the smallpox. Vaccinia, however, displays much milder symptoms. The exact origin of vaccinia is not known; it has been cultured in labs for years but there are no records of its original isolation. One hypothesis is that it might be a hybrid of the variola and the cowpox virus. It is, however, difficult to determine the origin because there are many strains of vaccinia in different labs which have different biological properties (Henderson et al. 1999). Vaccinia, a brick shaped virus, is one of the largest DNA viruses. It contains a 190 kB linear double stranded DNA genome that codes for 200- 250 genes. Half of these genes encode immediate and delayed early genes. Vaccinia is a unique virus as it produces two different kinds of virions which can spread the infection (Roberts et al. 2009). Furthermore vaccinia causes lysis of the host cell via necrosis. Though smallpox has been eradicated the potential role for variola, of which there are still stocks present, in the light of bioterrorism has kept an interest in vaccinia research (Ensirink et al 2002). Additionally, due to the fact that vaccinia is able to accept up to 25 kB of foreign DNA, it is popular in genetic engineering. In this regard, vaccinia is mostly used in oncolytic virus research (Kirn et al. 2009). 3.1.1 Disease Vaccinia infection occurs upon vaccination with a live vaccine against smallpox, which is nowadays no longer used as the disease is considered to be eradicated. 95% of the vaccinated individuals displayed full immunity for 5-10 years. The vaccine does not cause many severe side effects although there are some complications that could arise depending upon the immune status of the vaccinated individual (Lee 2012). These complications were in some cases lethal resulting in an average of 1 death per one million vaccinated individuals. In most vaccinated individuals mild pain would occur for about 7-10 days post vaccination at the spot of the vaccination, where a rash could also appear. The mild pain might be accompanied by regional lymphadenopathy and a low grade fever (Enserink et al. 2002). Due to these mild symptoms 1/3rd of the vaccinated individuals missed days of school or work hence the vaccine had an economical impact. The most common adverse effect was the acute vaccinia syndrome, which is characterized by fever, headache, mylagias and fatigue (Belongia et al. 2003). More severe side-effects included progressive vaccinia that led to metastatic infection of organs, necrosis of the skin around the vaccinated site and could also be fatal especially in individuals with a T cell deficiency (Cono et al. 2003). Children younger than 15, with eczema, are predisposed to post vaccination eczema vaccinatum, which can lead to hospitalization and at times to death. In even younger children microglial encephalitis and post vaccinial encephalopathy can occur resulting in 29 | P a g e fever, seizures and coma. Adults may develop a less severe CNS complication namely demyelination (Lee 2012). The virus can spread from the vaccination site, where it is shed, up to 21 days post vaccination. Consequently it is advised to cover the vaccination site. Furthermore the virus can drain from the primary site to the eyes, eyelids, nose and peritoneum, where it can cause inflammation. mild Nowadays laboratory workers and medical personnel Figure 7. Life cycle of the vaccinia virus. A. Entry of EV and MV particles is principally initiated by the induction of macropinocytosis resulting in the endocytosis of the virions. Here the fusion complex is activated by the are at risk of contracting vaccinia due to the fact that it is increasingly used progressive acidification of the phagosome, either directly or after the first for foreign gene transfer membrane of the EVs has been dissolved, allowing the viral core to be released research (Cono et al. 2003). into the cytoplasm. This acidification is not always necessary; it depends on the the perinuclear region where it replicates and is transcribed within replication 3.1.2 Infection mechanism Vaccinia has a broad cell type factories. The core and associated proteins are enclosed in a membrane of tropism that depends on strain which the origin has not been elucidated yet and this event leads to the and more in particular on the strain. B. Once the core has been released, it travels on microtubule towards formation of MVs. Here the assembly halts for many particles, which are then released upon cell lysis. Some particles continue on microtubules towards the post entry steps rather than the site where they obtain their second membrane. This has been suggested to entry either be of endosomal or trans Golgi origin. Obtaining a second membrane different vaccinia virions; the leads to the generation of EV, which can be divided into EEVs and CEVs. The mature virions (MV) and the former are propelled towards neighboring cells by an actin tail whereas the latter are released via exocytosis and allowed to infect more distant cells. Adapted from: Schmidt et al.2012 and Roberts et al. 2008 itself. There are two extracellular virons (EV). These do not share any epitopes hence use different factors for attachment (Bengali et al. 2012). MVs have been shown to bind to glycosaminglycans (GAGs) though this depends on cell type and strain type. Heparin sulfate and chondriotin sulfate have also been shown to be bound by MV epitopes. For EVs no cellular attachment factor has been identified. The 30 | P a g e main entry route of both virions is via endocytosis. To induce endocytosis, MVs and EVs initiate macropinocytosis. Macropinocytosis is the result of actin rearrangements which cause large amounts of fluid to be taken up into the cell. This was shown in several cell lines such as epithelial cells as well as monocyte-derived DCs. In CHO cells, however, the entry of MVs seemed to be independent of macropinocytosis. For EV, the virions which have a double membrane, the outer membrane needs to be lost in order to fuse with the endocytic membranes. This fusion is mediated by an entry/fusion complex consisting of 12 different viral proteins, the exact molecular details and architecture of which are currently unknown. The complex is, however, solely necessary for the virion fusion and it does not interfere with the binding process. Several strains require acidification to activate this complex while others are able to fuse in neutral conditions. Nonetheless an acidic environment as well as the exposure to GAGs is needed to disrupt the second membrane of the EV virions (Figure 7 A) (Schmidt et al. 2012). There is also a report stating that EVs enter the cell via fusion with the plasma membrane after they have shed their second membrane outside of the cell (Roberts et al. 2008). Once the core, containing the viral structural proteins, the compact DNA genome and the transcription enzymes, enters the cytoplasm, it is transported by microtubules to the perinuclear region where the replication factories are established. The vaccinia virus replicates entirely in the cytoplasm. Here partial uncoating allows for early mRNA transcription. The immediate and delayed early genes are the first to be transcribed and play a part in the replication of the viral DNA as well as in helping the virus to evade the immune system by manipulating the host cell to the advantage of the virus. Intermediate genes, of which there are relatively few compared to the large amount of immediate and delayed early genes, are subsequently transcribed. These genes encode regulatory proteins that drive the late gene expression, which are involved in the assembly of the novel virus particles as well as enocde the enzymes that are packed within the new virions. Next the virions are formed starting with the formation of the immature virion form. The viral DNA and other core components are packed in a single membrane vesicle, the origin of which is still unknown (Roberts et al. 2008). Aggresomes, which normally retain misfolded proteins, have been suggested to resemble the vaccinia replication factory (Wileman 2006). Proteolytic cleavage of the core proteins subsequently leads to the genertion of the brickshaped intracellular mature virus (IVM) also called MV. At this point the majority of virions halt their assembly process and the IVMs are released upon cell lysis. The IVMs which do continue morphogenesis are again transported by microtubule towards the site where they obtain their second membrane forming an intracellular enveloped virus (IEV). The formation of this second membrane has not been elucidated but it is thought that it has either an endosomal or a trans golgi origin. Subsequently they are transported to the periphery on microtubules. To reach the plasma membrane they must cross a barrier, the cortical actin. This is achieved by inhibiting RhoA signaling. The outer membrane of the IEV can thus fuse with the plasma 31 | P a g e membrane and an actin polymerization cascade is induced underneath the IEV, which propels the cell associated enveloped virus (CEV) away from the cell to infect neighboring cells. IEV can also leave the cell via exocytosis creating an extracellular enveloped virus (EEV) (Figure 7 B). EEV can spread to either adjacent cells but can also spread through the blood and infect distal cells. IVM and CEV/EEV have different viral proteins on their membranes and are hence structurally, antigenically and functionally diverse. CEV/EEV, also called EV, contain a host-derived lipid membrane and can therefore subvert the complement allowing it to travel to distant sites transported by the blood (Roberts et al. 2008). They mediate spread of the infection within the host while IVM are thought to mediate host-host infection. Most research on vaccinia has been done in immortalized epithelial cells however it is known that vaccinia prefers infection of polarized cells (Schmidt et al.2012). 3.1.3 Oncolytic virus Vaccinia is an ideal virus to use for oncolytic purposes and it has been shown that it can be used to treat skin tumors in xeroderma pigmentosum mouse models (Brun et al. 2012). It replicates and lyses the host cell quickly, and infected cells are already destroyed 48-72 hours post infection. Furthermore they have a broad tumor tropism that allows them to be used in the combat of various tumors. The virus can be made specific to tumor cells by deleting certain genes such as the thymidine kinase and/or VGF, which are both overexpressed in tumor cells and these will therefore be good hosts for the handicapped virus to retain its full ability. Additionally viral replication and spread are both linked to the activation of the EGFR-Ras pathway which is a pathway frequently activated in human cancer cells. A great advantage of vaccinia virus is that they are able to travel systemically through the blood. They can do this as EEV virions because this form is able to reach distant sites in the body without being targeted by the immune system (Kirn et al. 2009). Normally more IMV are produced but a mutation in the vaccinia genome can shift this balance towards a higher production of EEV increasing the beneficiary systemic traveling characteristic. Individuals pre-vaccinated with vaccinia, however, remain problematic as they contain neutralizing antibodies (nAbs) targeted against the virus. To overcome this problem in these individuals alternative delivery routes are explored which could evade these nAbs. For example different virion carriers are looked at which could aid in the evasion of these nAb. Temporarily suppressing the immune system has also been suggested as a method to deal with the virus being targeted for destruction. (Reinboth et al. 2012). 3.2 Vaccinia and autophagy The link between vaccinia infection and autophagy has not been well characterized. Considering that this virus replicates in the cytoplasm, one would imagine that autophagy will be induced as part of the cell immune response. This is, however, not dominantly visible as vaccinia virus potently infects 32 | P a g e its host cells. There are several viral proteins that could potentially be involved in the inhibition of autophagy. Some of these have not been investigated for their direct interaction with autophagy, such as N1, and others such as E3 have been implicated to be involved in blocking autophagy (Orvedahl et al. 2008; Cooray et al. 2007; Grahan et al. 2008). 3.2.1 Replication IVs are enclosed within a membrane of which the origin has not been fully elucidated. Not only is the origin under debate, there is also contradicting literature concerning whether these virions have a double or a single layer membrane though the single membrane theory takes the overhand. At first autophagy was proposed as a potential origin for the replication factory due to the double membrane characteristic. This was, however, disproved as Atg5-/- cells were shown to contain the replication factories and fully sustained vaccinia replication. Conformation was obtained by knocking down Beclin 1 proving that autophagy is dispensable for vaccinia replication (Zhang et al. 2006). 3.2.2 PKR pathway Vaccinia leads to rapid cell death and therefore it would be unlikely that vaccinia would induce autophagy upon infection as it has no need to prolong the host’s life span. Inhibiting the autophagy process would be more important for the virus as it would aid in evading destruction of the virions as well as it would reduce the host’s immune response. Autophagy has been shown to be induced by the PKR/eLF2α pathway, which also mediates host shut off. This pathway, which is universally blocked by viruses to evade autophagy, is actively inhibited by two different vaccinia viral proteins: E3 and K3 (Orvedahl et al. 2008). The former consists of an amino terminal Z DNA binding domain and a carboxy terminal dsRNA binding domain. E3 is essential for the pathogenicity contributing to vaccinia neurovirulence. With the dsRNA binding domain it interferes with the dsRNA mediated activation of PKR as well as with its dimerisation (Myskiw et al. 2011). The Z DNA binding domain has been suggested to block the recognition of viral nucleic acids by endosomal TLR sensing through the inhibition of autophagy. The exact mechanism how this domain would interfere with autophagy is, however, unknown. It would be interesting to see whether any proteins involved in autophagy can interact with this domain. Nonetheless the dsRNA binding domain and its ability to interact with PKR as well as the fact that E3, in primary DCs, is an antagonist of the innate immune response suggest that this protein could be involved in the inhibition of autophagy activation (Cao et al. 2012). Furthermore K3, which mimics the 88 amino acids of the N-terminus of eLF2α, blocks the PKR kinase catalytic site and consequently the intraction with its substrate, eLF2α (Bahar et al. 2011). These two proteins give the vaccinia virus two distinct ways via which the PKR pathway could be blocked indicating that this inhibition of autophagy and host shut down is of high importance for the virulence of the virus. 33 | P a g e 3.2.3 Bcl-2 like viral proteins Vaccinia also encodes several proteins such as N1, K7, A52 and B14 which are Bcl-2-like proteins because they all contain a Bcl-2 like fold. None of these have been studied in the context of autophagy. It is, however, known that viral Bcl-2-like proteins from other viruses can have the ability to bind to Beclin 1 and subsequently inhibit formation of an autophagosome. A52 and B14 have been shown to inhibit NFκB rather than apoptosis, which is mainly inhibited by N1 (Cooray et al. 2007; Grahan et al. 2008). N1 is conserved in most vaccinia strains with the exception of the modified Ankara virus, which contains a truncated version of the protein. The viral Bcl-2 like protein is able to regulate inflammation favoring the virus. Additionally it modulates both NK response as well as the activation of lymphocytes. Knocking down N1 did not affect the replication of the virus but the virus was cleared more efficiently by the immune system. It also resulted in an increase of NK cells and a decrease of T cells infiltrating the area of infection (Jacobs et al. 2008). Hypothetically if N1 is indeed involved in the suppression of autophagy inhibiting N1 would result in an increase in autophagy. The extent of the increase might not be very large as there are probably additional, both experimentally proven as well as less known, pathways suppressing autophagy. The hypothetical increase in autophagy and hence increased virus destruction are in line with the fact that without N1 the virus is more easily cleared. However, the decrease in T cells involved upon infection with the N1 lacking vaccinia contradict the involvement of N1 with autophagy. This is due to the fact that less T cells would indicate less viral epitopes being presented at the cell surface in MHC. An increase in autophagy would, conversely, result in increased degradation of the virus and hence a higher availability of viral epitopes for MHC loading. With a higher concentration of these epitopes one would expect an increase in T cells infilitrating the infected area. Considering this it could very much be the case that N1 does not have anything to do with autophagy. The inhibition of viral clearance by N1 might thus be due to a completely different mechanism. Nonetheless, during influenza virus infection autophagy is not involved in MHC II antigen presentation (see 5.2.1), hence perhaps the same holds true for vaccinia. 3.2.4 Atg12-Atg3 conjugate A strong link between vaccinia infection and autophagy was made when Maloughney and colleagues discovered that upon infection with this virus no autophagosomes are formed. Moreover, amino acid deprivation-induced autophagosome formation is abolished during a vaccinia infection. This indicates that vaccinia actively inhibits cellular autophagy. Infection did however cause high levels of the lipidated form of LC3. These LC3-II puncta were different from the ones seen during starvation as they appeared as aggregates. LC3 lipidation depends on Atg7 and in part on Atg 5. Lipidation of LC3 during vaccinia infection did not require these two factors as the corresponding knockout cells still displayed the increased levels of LC3-II. This modification, however, depends on Atg3. Indeed 34 | P a g e knocking down Atg3 resulted in loss of the increased LC3-II. Atg3 was shown to colocalise and to form a conjugate with Atg12. This conjugate was found near the viral factories suggesting that the virus is indeed responsible for the formation of this novel conjugate. The colocalisation of Atg12, Atg3 and the replication factory could, however, not be visualized at once as there were no commercial antibodies available which would make this possible (Moloughney et al. 2011). The exact method via which this conjugate is formed and hence causes inhibition of autophagy has not been elucidated yet. The conjugate was previously shown to be involved in mitochondrial homeostasis highlighting the possibility that vaccinia hijacks an endogenous system/pathway (Radoshevich et al. 2010). The Atg12-Atg3 conjugate by itself is, however, unable to lipidate LC3 and therefore another viral or perhaps cellular component is necessary. A candidate molecule would be an E1-like protein because Atg7 is not required and consequently a replacement for this molecule might be encoded on the vaccinia genome. A model showing the mechanism how vaccinia may inhibit autophagy via the Atg12-Atg3 conjugate is depicted in figure 8. It has been proposed that this conjugate would covalently link the E2-like and the E3-like enzymes involved in autophagy and thus short circuit the LC3 lipidation system making use of a as of yet unknown viral protein. In parallel, the absence of Atg12-Atg5, as Atg12 now binds to Atg3, will hamper the formation of the Atg12-Atg5-Atg16L essential for the autophagosome biogenesis. These two events would act synergistically in blocking autophagy (Moloughney et al. 2011). Figure 8: Proposed model for Atg12Atg3 conjugation and subsequent inhibition of autophagy. It has been suggested that the novel Atg12-Atg3 conjugate, generated by two yet unknown E2- and E3-like enzymes, shorts circuits the LC3 lipidation process. This along with the fact that possibly the formation of this novel conjugate abrogates the formation of the Atg12Atg5-Atg16L complex, inhibts autophagy. Adapted from: Moloughney et al. 2011 35 | P a g e 3.3 Future directions Autophagy is definitely inhibited by the vaccinia virus infection as was shown by Maloughney and colleagues upon the discovery of the Atg12-Atg3 conjugate, which abolished formation of autophagosomes. As mentioned in the previous section (2.2.4) LC3 lipidation could not be accomplished by this conjugate itself and hence it was suggested that perhaps an virally encoded E1 like enzyme might be involved in this process (Moloughney et al. 2011). In addition, an E2- and an E3like enzyme are also necessary and might perhaps also be present in the viral proteome. If this is, however, not the case perhaps host factors are needed which are recruited by other viral proteins. To determine which viral proteins are essential for the formation of the Atg12-Atg3 conjugate as well as for the subsequent lipidation of LC3, one could look at different mutant strains generated by reverse genetics and identify whether there are specific mutations that lead to a phenotype where the increased LC3-II production during infection in the absence of autophagy is lost. This is quite a laborious task considering the fact that there are around 200 genes in the vaccinia genome. The formation of the Atg12-Atg3 complex could be monitored by fluorescence microscopy but this is currently not possible because there are no commercial antibodies available which allow the detection of Atg3 and Atg12 (Moloughney et al. 2011). To this aim and for other possible experimental approaches, it would be important to generate specific antibodies against these two proteins as well as against the conjugate. If the knockout of a viral protein is found to alter this LC3 lipidation in the absence of an autophagy phenotype, its binding to the conjugate could be checked by pull down assays. The Atg12-Atg3 conjugate has also been shown to be involved in maintaining mitochondrial homeostasis. As a result, it cannot be excluded that a viral component hijacks a host pathway involved in the function of the conjugate. Nonetheless, the inhibition of autophagy via the formation of the Atg12-Atg3 conjugate appears to be very effective and consequently this is most likely the main way how vaccinia blocks autophagy during infection. This observation would suggest that other ways used by vaccinia to inhibit autophagy would be of minor importance. Nonetheless these alternative mechanisms of inhibiting autophagy are still interesting to be investigated more in depth. The Bcl-2 like proteins might not be the most interesting group of potential autophagy inhibitors. It is in my opinion unlikely that N1 interacts with autophagy mediators. This is due to the fact that if N1 is knocked down, apoptosis is activated upon infection (Jacobs et al. 2008), since it is an anti apoptotic molecule. If N1 would additionally also be an inhibitor of autophagy this knock down would counteract apoptosis. A high level of autophagy could tilt the balance towards apoptosis, however, this seems unlikely since the Atg12-Atg3 conjugate inhibiting autophagy would still be in place. Hence the idea that it blocks autophagy and the knockout study showing increased apoptosis are not in line with one another. Additionally the 36 | P a g e decrease of T cells in the infiltrate of immune cell post infection of a N1 knock out virus would suggest that autophagy is not important in presenting viral antigens on the surface, which is possible as it is also seen in influenza but more often autophagy is considered to play a role in this process. Nonetheless if it does have a role in blocking autophagy knocking down N1 might not have any significant effect on restoring autophagy since the formation of the Atg12-Atg3 conjugate will overpower it in blocking autophagy. With these considerations in mind, it would be still interesting to look whether Beclin 1 is a binding partner of one of the viral proteins containing the Bcl-2-like fold. If an interaction with Beclin 1 is found, then these Bcl-2 like proteins would most likely play a minor role in the inhibition of autophagy upon infection, but they could become important if the cells can somehow inhibit or counteract the effects of the Atg12-Atg3 conjugate. It will be hard to test the individual effect of the Bcl-2 like proteins on autophagy during vaccinia infection, but this could be done if the Atg12-Atg3 conjugate formation is blocked. This is considering the fact that knocking out either protein would have an effect on autophagy itself as well as the fact that no other proteins which are involved are known very tricky. One could possibly make a form of Atg12 or Atg3 which is not able to bind to the other but does maintain its function in autophagy. These mutants could subsequently be transfected into a cell where the wt versions have been knocked down. To my knowledge the exact interaction between these two proteins has however not been elucidated yet hence this would provide the first step. Additionally if an essential viral protein is found associated with the Atg12-Atg3 complex, it could be used to abolish its function by depleting it or mutating it and the effect of N1 or other viral proteins could be analyzed in a infection context where the principal mechanism used to inhibit autophagy is not present. Furthermore the relevance of the PKR inhibition and thus the block of autophagy also requires additional research. Again the absolute contribution to autophagy inhibition is most likely not very large compared to the Atg12-Atg3 conjugation inhibitory pathway. Like the Bcl-2-like proteins, it will be hard to determine the inhibitory effect of E3 and K3 by themselves, which will be even harder if the Bcl-2-like proteins are also involved in blocking autophagy. The Z DNA binding domain of E3 has been proposed to inhibit autophagy as it interferes with the recognition of viral nucleic acids; the exact process, however, is not known (Cao et al. 2012). Perhaps this domain binds the viral nucleic acids and thus shields them from being recognized by the autophagy machinery. This will allows interfering with recognition without directly interfering with the autophagy machinery. Nonetheless it could also possible that this domain interacts with Atg proteins and does indeed inhibit the machinery. Whether any of the known Atg proteins interact with this domain could be tested with the FISH technique or co-immunoprecipitation, or perhaps on an affinity column containing the 37 | P a g e binding domain. Identification of a potential Atg binding partner will allow elucidating whether the domain is directly involved in inhibition of autophagy. In conclusion, the most promising pathway via which vaccinia inhibits autophagy preventing destruction of the virus as well as presentation to the immune system is via the Atg12-Atg3 pathway. The virus relatively quickly causes post infection cell death via necrosis, which might be aided by the fact that autophagy is blocked. Nevertheless this principally serves the purpose of releasing the IVMs inside the host for progressive infection. Although this is most likely the main mechanism of autophagy inhibition, large parts of the pathway remain to be elucidated along with potential minor inhibitory pathways. 38 | P a g e 4. Coronaviruses 4.1. Introduction Coronaviruses (CoV) became public concern when the severe acute respiratory syndrome (SARS) CoV surfaced in 2002 resulting in worldwide morbidity and mortality. Before the SARS emergence, CoV were mostly a veterinary health issue. CoV A viral particle diameters range from 80 to 120 nm and are enveloped, pleimorophic or spherical (Belouzard et al. 2012). The name corona is derived from the appearance of the virus under the EM as it has a crown structure due to trimers of the spike (S) protein on the surface of the virion. They are single stranded positive sense RNA viruses which containing a 5’ cap and have a length B between 26.2 characteristic and places 31.7 them kb. This among the longest RNA viruses. The genome, consisting of 6 to 10 open reading frames (ORFs), is enclosed in a helical nucleocapsid packed in a host derived lipid bilayer (Perlman et al. 2009). The replicase proteins are encoded by the first ORF, making up two-thirds of the genome. The structural protein genes, ultimately present in the virion envelope, have a conserved order in the last third of Figure 9: Coronavirus genome and structure. A. 3 different CoV the ORF: the hemagglutinin esterase (HE), genomes containing the in red colored ORF1 and the structural which is not encoded for by all CoV, the S proteins (HE), S, E, M, and N. B. The structure of CoV (not drawn in scale). Adapted from Belouzard et al. 2012 protein , the envelope(E) protein, membrane (M) protein and nucleocapsid (N) protein (Figure 9). There are four different genera of CoV, both based on serology as well as on genetic studies: the alpha-, beta-, gamma- 39 | P a g e and delta- CoV. Although the fundamental steps in the viral life cycle are conserved amongst the different CoV genera, they use different receptors to initiate the infection life cycle (Belouzard et al. 2012). 4.1.1 Disease CoV mainly causes intestinal and/or respiratory infections. Often CoV do not cause severe disease in humans however they may manifest systematically (Kahn et al. 2005). 30% of all human colds can be attributed to CoV. It is, however, difficult to culture CoV and hence to connect them with other diseases (Engleberg et al. 4th edition). In humans the clinical effects from CoV can range from the common cold, as mentioned before, to pneumonia and acute respiratory distress syndromes. The latter was the case when SARS, a beta-CoV, emerged causing severe respiratory disease with a 10% mortality rate (Gu et al. 2005). Furthermore the human CoV–NL63, an alpha- CoV, also leads to severe respiratory tract disease (Wenhui et al. 2007). Similarly to most of the viruses, immunocompromised individuals as well as newborns are more likely to develop severe pulmonary diseases even from mild penetrant CoV (Kahn et al. 2005). The appearance of SARS also revealed the ability of CoV to adapt from one species to another. This was discovered as SARS had characteristics from both mammalian and avian CoV (Engleberg et al. 4th edition). This indicates that CoV can undergo adaptations continuously during their life cycle and they can thus potentially cause severe human diseases. It remains clear that CoV have a high economic impact because they affect a large number of mammals, including dogs, cats, horses and other life stock and birds. In these animals they cause respiratory and enteric diseases, and less commonly can also lead to hepatitis and neurologic illnesses (Belouzard et al. 2012). Infection bronchitis virus (IBV) for example, is a gamma- CoV that can lead to extreme economic losses in the poultry business (Cottam et al. 2011). Transmissible gastroenteritis virus leads to diarrhea in pigs like bovine CoV does in cattle (Saif et al 2010; Kim et al. 2000) Infection with the mouse hepatitis virus 59A (MHV) strain, a beta-CoV, can result in encephalomyelitis. This model is often used for research regarding multiple sclerosis (Tsuhako et al. 2009). 4.1.2 Infection mechanism The infection cycle starts as the virion interacts with a specific protein/receptor on the surface of the target cell triggering fusion. The S protein, a type 1 transmembrane protein, is crucial for these two steps playing both a role in the tethering to the target membrane as well as the subsequent fusion of the virion with it. The S protein is highly glycosylated and assembles in trimers. It consists of 2 domains: the S1 domain, at the N-terminus, is important for receptor binding, and the S2 domain at the C terminus, which is highly conserved and is involved in membrane fusion (Bosch et al. 2003). The 40 | P a g e different CoV make use of different receptors to tether to the target surface making the S protein the determinant for cellular tropism. MHV makes us of the adhesion molecule CEACAM1, which is part of the Ig superamily. Most alpha-CoV, such as the feline and canine CoV, bind to aminopeptidase N which is also called CD13 and is present at the apical domain of epithelial cells of the enteric and respiratory tracts. Figure 10: The life cycle of the CoV. CoV bind to their preferred receptor While these different CoV recognize and via a conformational change of the S protein fuse either directly with the same receptor, they interact the plasma membrane or after they are endocytosed. After fusion, the with different amino acids. These virions dissociate and release their genomic RNA into the cytoplasm. Here binding domains are located in a two large precursor polyproteins are made that are subsequently processed non-homologous region. Sialic acid by viral proteases. Replication-transcription complexes anchored into DMVs are formed. Structural proteins are made from nested subgenomic RNAs (SA), the known receptor for and the genomic RNA is replicated. Subsequently the structural proteins influenza, is also used as binding and genome are assembled in the ERGIC. They form a particle by budding interface by the IBV and the bovine into the ERGIC and hence obtain an envelope. Lastly they are transported CoV. The SARS and human CoV through the Golgi and eventually are released via exocytosis into the extracellular space. Haan et al. 2008 NL63 both converting bind enzyme angiotensin 2 (ACE2). Furthermore SARS as well as IBV interact with DC-SIGN, which is a C-type lectin receptor on macrophages and DCs known to be highjacked by HIV, because of the heavy glycosylation of the spike protein. Once the virion is attached to the membrane, it can enter the cell by either fusing directly with the plasma membrane or by fusion after it is internalized via endocytosis. Both situations can take place with MHV though fusion is principally triggered by binding to the receptor. IBV, on the other hand, is endocytosed and fusion is induced by the lowering of the pH in the endosome. Fusion is the result of a conformational change of the S protein and involves proteolytic cleavage, either by a viral or a host protease. This change brings the fusion peptide, an apolar 15-25 amino acid long region predicted to be in the S2 domain, in close contact with the transmembrane domain facilitating fusion. Once fused, the virion dissociates and releases its RNA genome into the cytoplasm (Belouzard et al. 2012). The 41 | P a g e entire processing and replication process takes place in the cytoplasm. Here two large polyproteins are synthesized, which are cleaved by viral proteases into 16 non structural protein (nsps). These nsps are essential for the formation of the RNA replication-transcription complex (Perlman et al. 2009). These replication complexes are anchored into DMVs, of which the origin has been the subject of much debate as will be discussed later in this chapter (Reggiori et al 2008). DMVs are a very elegant way via which the virus ensures that the viral RNA is protected from cellular defense. Formation of both these DMVs and the replication translation complexes leads to the translation of the structural proteins from subgenomic nested RNA. This organization permits the RNA to have a poly-A tail and a cap to resemble cellular mRNA so that it can make use of the host translation machinery. (Perlman et al. 2009). The genome is replicated by the viral RNA-dependent RNA polymerase, which is very error prone. This tendency to make mistakes relates to the fact that CoV relatively easily obtain mutations. At times, this can result in interspecies jumps. Indeed closely related CoV were discovered in distantly related animals indicating that this was caused by recent interspecies jumps (Belouzard et al. 2012). Additionally homologous recombination has been shown to occur in CoV due to their large RNA size. Homologous recombination would result in a strain adapting novel characteristic from another CoV upon co-infection (Thor et al. 2011). Newly made genomic RNA is subsequently assembled with the structural proteins at the ER-Golgi intermediated compartment (ERGIC) (Ruch et al. 2012). The newly formed virions bud inwards into the ERGIC forming an enveloped virion that is extracellularly released via exocytosis (Boscarino et al. 2008) (Figure 10). 4.2 Coronavirus and autophagy DMVs are the hallmark of a CoV infection. The origin of these DMVs has been much debated. Due to their appearance, one hypothesis was that perhaps CoV were derived from autophagosomes. Experimental exploration of this hypothesis resulted in some contradictory results on whether autophagy machinery is important for CoV-induced DMVs formation and hence replication. DMVs seem to be derived from the ER as ER markers are found on DMVs as well as because they are found close to the ER and occasionally even attached to it (Prentice et al. 2004; Zhao et al. 2007; Haan et al. 2008). The ER origin coincides with autophagosomes, which also appear to be of ER origin, though they can also be derived from the mitochondrial outer membrane (Tooze et al. 2010). Furthermore DMVs were shown to be decorated with LC3, an autophagy marker protein. Denison and collegues additionally showed that Atg5, another important autophagy protein, was necessary for the formation of DMVs in murine embryonic stem cells (Prentice et al. 2004;). This latter finding was however contradicted by a paper in which Atg5 was shown to be dispensable for DMVs formation in primary macrophages and low passage primary embryonic murine fibroblasts (Zhao et al. 2007). This 42 | P a g e would indicate that autophagy is not at all required for CoV replication in these cells. Further discrepancy arose as some labs were able to show LC3 colocalizing with the DMVs while others could not (Prentice et al. 2004; Zhao et al. 2007; Haan et al. 2008). 4.2.1 Highjacking LC3-I LC3 is present on the membrane of the DMVs in the LC3-I variant, which unlike the LC3 form present on autophagosomes, is not lipidated (Reggiori et al. 2010). This explains why some groups were able to find the colocalisation while others were not. They used different detection methods as some utilized antibodies that recognized all LC3 while others made use of autophagosome specific GFPLC3, which is in the LC3-II form and consequently does not colocalize with DMVs (Prentice et al. 2004; Zhao et al. 2007; Haan et al. 2008). LC3-I knock down results in the severe impairment of viral replication showing that LC3-I is indeed important for CoV life cycle. LC3-I is converted to LC3-II by Atg7, amongst other Atg proteins. Hence since the DMVs have non lipidated LC3 in their membrane there should be no need for Atg5 nor for autophagy. LC3-I is in fact not a functional autophagy protein unless it is transformed into LC3-II. This discards the hypothesis that DMVs are autophagosomes. Nonetheless this does not reveal the origin of these DMVs. There is, however, another structure in the cell that also has LC3-I onto its membranes, namely the EDEMosomes (Reggiori et al. 2010). 4.2.1.1 The ERAD tuning pathway EDEMosomes are part of a mechanism of control of the ER-associated degradation (ERAD) pathway. ERAD ensures quality control of proteins in the ER; misfolded proteins are dislocated into the cytosol and degraded by the ubiquitin-proteosome pathway. Because proteins that are still in the process of folding under normal conditions also attract the ERAD machinery, the ERAD factors need to be tightly regulated in order to avoid that these intermediate proteins are degraded. One of the ways of control is VIA ERAD tuning. During this process, the ERAD factors are selectively removed from the ER. EDEM1 and OS-9 are a couple of these ERAD regulators and they are sorted out of the ER in EDEMosomes. EDEMosomes are 200-800nm large single membrane vesicles, which are decorated with LC3-I. Furthermore EDEMosomes are formed in the ER in a COPII protein coat-independent mechanism. They subsequently deliver their content to endosomes for degradation. The exact mechanism of this disposal, however, remains unknown (Bernasconi et al. 2011). 4.2.1.2 DMVs and EDEMosomes DMVs have also been shown to contain EDEM1 and OS-9 suggesting that CoVs hijack the EDEMosomes. These two ERAD proteins, however, are not essential for MHV replication (Reggiori et al. 2010). Trapping ERAD proteins might be beneficial later during infection when the ER stress is increased due to the high production of viral proteins. This would normally turn on the ERAD 43 | P a g e pathway and thus degrade viral proteins, which can be minimized by trapping the ERAD proteins in a different location (Reggiori et al. 2011). DMVs will also need to be modified EDEMosomes considering the fact that EDEMosomes normally fuse with endosomes. As DMVs are the replication site of CoV, they must somehow stop the vesicle from fusing with the endosomes. The exact mechanism how EDEMosomes become DMVs has not been solved nor has the exact function of LC3-I in the formation of these vesicles been unveiled. 4.2.1.3 Models There are a few proposed models for the role of LC3-I in CoV infection as well as for the formation of DMVs from EDEMosomes. LC3-I is part of a complex including SEL1L and either OS-9 or EDEM1 during steady-state delivering the ERAD proteins to the endosomes. It is however not clear whether LC3-I binds directly or indirectly to Sel1L. This suggests that LC3-I would be part of an EDEMosome cargo receptor (complex) (Bernasconi et al. 2012). A way for CoV to hence hijack the ERAD tuning machinery and form DMVs would be to hijack this cargo receptor. This could perhaps be done by a viral nsp. LC3-I could on the other hand participate in ERAD tuning/viral replication by attaching the DMVs and EDEMosomes to the microtubule network. MHV replicative structures indeed have been shown to move along microtubules to concentrate in the perinuclear region. Nonetheless microtubules are not crucial for MHV replication and therefore LC3-I must have another key function in CoV replication (Reggiori et al. 2011). The fact that LC3-I seems to associate with the cargo receptor SEL1L, makes the hijacking theory by one or more viral proteins an attractive possibility. How are the CoV-induced DMVs derived from the single membrane EDEMosomes? There are two models that still need to be explored experimentally (Figure 11). The first involves the reticulovesicular network (RVN) that is formed upon SARS infection. This modified ER has both convuleted membranes as well as many interconnected DMVs. The mechanism of this model is nonetheless unclear. In the second model DMVs could be the result of invaginations and subsequent fusion of the extremities of a single membrane vesicle, i.e. an EDEMosome, regulated possibly by a viral protein (de Haan et al. 2010). Further studies are needed to elucidate both the role of LC3-I as well as the role of viral and host proteins in the formation of DMVs from the ER. Though LC3-I once lipidated is a protein involved in autophagy it is now clear that autophagy itself is not involved in the formation of DMVs and the replication of CoV. It does however show that proteins involved in autophagy can have additional and different role within the cell. 44 | P a g e Figure 11: Hypothetical models for the origin and formation of DMVs. DMVs could originate from RVNs as seen in SARS-infected cells where a large number of interconnected DMVs are formed (model 1). Another model is based on the inward budding profile of DMVs and these could form by invaginations and subsequent fusion of the extremities of this intermediate (model 2). This event could be mediated by viral proteins. Adapted from: de Haan et al. 2010 4.2.2 Induction of autophagy Autophagy has been shown to be induced upon infection with CoV through nsp6, a membrane spanning protein. Transfection of nsp6 introduction of IBV, SARS and MHV into cells resulted in the induction of autophagy via an omegasome intermediate. Omegasomes are domains in the ER that contain DFCP1, which is recruited to the ER upon activation of autophagy, and that recruit key autophagy proteins such as LC3, Atg5 and Atg14. The mechanism by which nsp6 induces autophagy does not rely on the classical inhibition of mTOR, induction of ER stress or activation of Sirun1. It is currently unclear how this viral protein leads to the formation of autophagosomes. It might be due to the enhanced production of Ptdlns(3)P which binds DFCP1, because higher levels of Ptdlns(3)P were measured after cell transfection with nsp6. Nsp6 could also mimic Atg14 and directly recruit Beclin 1 to the ER. Furthermore it could inhibit Jumpy. Jumpy is a phosphatase that dephosphorylates Ptdlns(3)P into PtdIns inhibiting autophagy. The ability to induce autophagy was however shown by introducing solely nsp6 into the cells in absence of a CoV infection. This is not a physiological situation hence it does not directly show that nsp6 induces autophagy during virus infection. Autophagy could be triggered during virus infection by either RNA being present in the cytosol, which could be sensed by the immune system, or perhaps viral protein complexes might induce autophagy (Cottam et al. 2011). If nsp6 is part of a complex during infection this might also interfere with its ability to induce autophagy. However if nsp6 indeed induces autophagy during viral infection this would not seem beneficial for the virus. Considering the fact that autophagy could degrade viral particles, induction of this pathway would not be advantageous. Conversely, perhaps nsp6 makes use of autophagy to target certain immunomodulatory proteins, which are synthesizes in the ER towards the autophagosome degrading them and consequently decreasing the host immune response. Alternatively, as for the VZV, the virus needs to induce autophagy to ensure a longer life span of the host cell considering the fact that they interact with the ERAD machinery decreasing the clearance of 45 | P a g e Figure 12: ERAD tuning and highjacking by coronaviruses. The ERAD pathway is important in the clearance of misfolded proteins. Under normal conditions the ERAD factors need to be tightly regulated preventing premature intermediate proteins from being degraded. This is also called ERAD tuning, where certain ERAD factors are selectively removed from the ER. EDEM1, one of these ERAD regulators, along with OS-9, is sorted out of the ER in EDEMosomes. This is done by the cargo receptor SEL1L, which simultaneously binds these ERAD factors and LC3-I. The exact binding of the latter has not been determined yet. The EDEMosomes subsequently deliver their content to the endosome/acidic compartment for degradation. CoV highjack these vesicles forming DMVs, where they replicate, through a mechanism that still remains unknown. Additionally CoV-induced DMVs do not fuse with endosomes/lysosomes hence they must somehow inhibit this step. Bernasconi et al. 2012 misfolded proteins which could result in cell death. These latter two possibilities would be beneficial for the virus. 4.3 Future directions There is not a clear link between autophagy and CoV. Autophagy was first thought to be hijacked by CoV, as the virus replicates on DMVs, which are morphologically similar to autophagosomes. However, the LC3 form decorating both membranes is different. While autophagosomes are decorated with lipidated LC3, DMVs have LC3-I, the non lipidated version, on their membranes. These DMVs were instead shown to be derived from EDEMosomes. This was suggested as both vesicles have the LC3-I on their membranes as well as both contain the ERAD regulators EDEM1 and OS-9. However it could still be possible that CoV hijack LC3-I and that the two ERAD proteins are actively recruited by CoV into the DMVs to shut down the ERAD (Reggiori et al. 2011). LC3-I has been shown to associate with SEL1L, a cargo receptor, and EDEM1 and OS-9 providing evidence for a CoV nsp hijacking of the cargo receptor and thus forming a vesicle with LC3-I on the surface (Bernasconi et al. 2012) (Figure 12). Further research is necessary to determine how LC3-I binds this cargo 46 | P a g e receptor, directly or via an adaptor protein, and whether one of the nsp of CoV is able to bind either SEL1L, another cargo receptor or perhaps binds the complete complex including LC3-I. The exact mechanism via which the DMVs are actually formed is also subject for further research. Whether this is via the RVN during SARS infection or whether RVN is only used for replication later in infection once the cell is no longer able to make isolated DMVs. To determine the latter it is key to look at whether CoV are also able to replicate within the convulated membranes. Another suggested model involves the pinching off of invaginations of single membrane vesicles perhaps by a viral protein. It would be good to start by investigating whether one of the viral proteins is in fact able to pinch off or cleave something off the ER. Shedding more light on these processes would help in understanding the exact mechanism via which CoVs manipulate the host cell to create their replication paradise (Haan et al. 2010). Again this is not a direct link between virus and autophagy, which is the main focus of this thesis. However it is important to note that proteins involved in autophagy seem to additionally be able to function in different cellular processes, in this case LC3 in a non lipidated form. Moving on to the autophagy relevant part of this section it is fair to conclude that autophagy has not been firmly linked to CoV. There is evidence that nsp6 induces autophagy, however, the experiments were done by introducing solely nsp6 into the cells (Cottam et al. 2011). Thus the autophagyinducing characteristic of nsp6 might not be present once the cell is infected with the entire virus. Nonetheless the paper brought to light some things that will be interesting to look at in future experiments. Nsp6 being a transmembrane protein was predicted to be important in the replication transcription complex as it would span the membrane of the DMVs. Additionally it was also shown to be present on the ER membrane as some C-terminal residues were glycosylated. This would mean that it would both induce autophagy and also be present on the DMVs independently from autophagy. There are, however, two different nsp6’s found which could be either two different ones or one could be post translationally modified or otherwise alternatively processed (Baliji et al. 2009). Perhaps one of these can induce autophagy while the other is present at the DMVs playing part in the replication of the virus. Either way it is important to explore whether nsp6 is the causative agent for autophagy induction when cells are infected with the entire virus or whether autophagy is induced via a completely different mechanism. If autophagy is indeed induced by nsp6, looking at how it is induced, as it is not via inhibition of mTOR or induction of ER stess is an elegant next step. Here one could look at Jumpy, Ptdnlns(3)P or whether it bind Beclin directly or perhaps induces the PKR pathway (Cottam et al. 2011). On a side note, IBV nsp2, nsp7 and IBV-induced overexpression of GADD34 result in antagonism of PKR. This is done by interacting with the phosphatase PPI resulting in less phosphoryalted eIF2α and by participating in the dephosphorylation of eIF2α respectively 47 | P a g e (Cruz et al. 2011). Though this has not been directly related to autophagy, it is known that the PKR pathway can stimulate autophagy thus interacting with proteins involved in this pathway counteracting the activity of PKR might also result in some inhibition of autophagy (Talloczy et al. 2006). Additionally the reason for autophagy induction, whether it is beneficial for the virus, would be an intriguing next step. Perhaps as mentioned before, induction of autophagy by nsp6 is somewhat specifically targeting immunomodulatory proteins to their degradation via autophagy. This raises the question how this autophagy is then driven to be more specific to the level that the virus benefits more than being destroyed. Again the virus could also simply need the autophagy to elongate the life span of its host so that it can replicate more. All together the relationship between autophagy and CoV has not been elucidated yet. These viruses do show that autophagy proteins, such as LC3, can participate in processes other than autophagy. Nonetheless one of the only links connecting autophagy and CoVs is nsp6 which seemingly induces it. This, however, needs to be validated in the context of functional CoV infection where other factors might influence nsp6 hence disturbing this potential role in inducing autophagy. Furthermore the PKR pathway also seems to be inhibited by IBV. This has not been investigated in the light of autophagy inhibition. Concluding only little has been revealed considering this virus and autophagy and more research is required in order to gain substantial knowledge in this part of the field. 48 | P a g e 5. Influenza 5.1 Introduction Influenza viruses are double stranded negative sense segmented RNA viruses which contain a genome with a size of approximately 10- 13.6 kilobases. There are three different types of influenza viruses, A, B and C, which are all very similar in structure. They, however, are quite diverse and show no cross reactivity with antisera, a characteristic that separates them from each other (Engleberg et al. 4th edition). Influenza virus C contains the smallest genome as it has 7 segments compared to the 8 segments found in influenza virus A and B. Each segment of RNA is packed by the viral nucleoprotein (NP) as well as by three viral polymerase proteins. The matrix protein surrounds the encapsidated RNA segments and is in turn packed in a host cell-derived membrane. Two viral surface glycoproteins, hemagglutinin (HA) and neuramidase (NA), are embedded in this membrane and play an important part in viral entry and exit, respectively. HA forms a homotrimer on the surface of the virion. The focus of this chapter will be the influenza virus A. The 8 influenza A virus RNA segments encode 11 different viral proteins (Palese et al. 2007). The largest three segments contain three RNA polymerase components as well as the polymerase basic protein (PBIF2), which promotes cell death. The smallest segment produces the nonstructural proteins, ns1 and ns2, which compromise the IFNmediated response by preventing innate recognition and support the nuclear export of packed viral RNA, respectively. The remaining segments encode HA, NA, NP and the two matrix proteins, M1 and M2. M2 is important in the uncoating of the virion which will be discussed in more detail in the next section (Gannagé et al. 2009). There are currently 16 different serotypes of HA and 9 serotypes of NA known allowing various combinations. Antigenic shift and drift are two processes important for influenza pathogenesis through the creation of novel HA/NA combinations (Engleberg et al. 4th edition). Antigenic drift results in the accumulation of mutations in the antigenic sites of either HA or/and NA. This happens in all influenza viruses but occurs more frequently in the influenza A virus, which is more prone to mutations. In a time span of 3-5 years the drift can give rise to a novel strain causing disease (Boni et al. 2006). Antigenic shift is what distinguishes influenza A from influenza B and C viruses. It involves the exchange of HA and NA genes from one virus to another. This process is also called reassortment and happens only in influenza A virus due to its ability to infect multiple species. It can cause serious disease as the immune system will not be able to recognize the virus, which has obtained novel surface markers, and may not be able to clear it successfully. Antigenic shift is the cause of many flu pandemics that have occurred in the past (Smith et al. 2009). 49 | P a g e 5.1.2 Disease The influenza A virus is the causative agent of the Spanish flu H1N1 pandemic in 1918 as well as for the more recent pandemics such as the 1957 Asian H2N2 flu, the 1968 Hong Kong H3N2 flu and the most recent swine H1N1 flu pandemic in 2009. This latter pandemic caused fewer deaths than the common flu however was classified as a pandemic due to the quick spreading (Smith et al. 2009). Nonetheless, influenza can causes severe respiratory diseases that are highly contagious through airborne droplets. Influenza clinical manifestations include fever, malaise, headache, chills and respiratory symptoms such as cough. It can lead to several complications ranging from pneumonia to encephalitis. During mild cases of influenza, the pathology displays affected respiratory epithelial cells with edema which show inflammation due to the infection. Additionally, mononuclear cell infiltrates are observed. More severe disease manifestations are caused by influenza damaging the tissues. In this case the lungs become hemorrhagic and airless as well as display necrotizing tracheobronchiolitis and bronchitis (Engleberg et al. 4th edition). This tissue damage and the virus itself cause lymphopenia as well as severe inflammation which can result in a cytokine storm and this could be lethal. It is thought that a cytokine storm caused the high number of deaths during the Spanish flu outbreak as well as during the H5N1 avian influenza A virus pandemic (Osterholm et al. 2005). This latter virus strain has been responsible for human deaths since 1997, having a mortality rate of 50%. It is characterized by a rapid progression to acute respiratory distress syndrome (Ma et al. 2011). Influenza causes thousands of deaths annually as well as enormous economic loss, tens of billions of US dollars, due to the time needed to recover from debilitating infections. Thus it also causes social disturbances (Gannagé et al. 2009). The influenza C virus is the least severe virus and is nonseasonal. In contrast, the influenza A and B viruses can cause the severe respiratory diseases described above and occur between October and April, peaking between December and March on the northern hemisphere (Engleberg et al. 4th edition). 5.1.3 Infection mechanism The influenza virus enters the lungs and infects cells of the respiratory tract such as nonciliated epithelial cells, which are commonly the first to be infected (Engleberg et al. 4th edition). A terminal SA linked to galactose is the preferred receptor for the influenza virus. Human viruses recognize a α2,6 linkage while avian influenza binds to a α2,3 linkage. Swine influenza can recognize both of them (Samji, 2009). The membrane protein HA binds to SA. HA0 is the precursor of HA. This precursor consists of HA1, which directly binds to the SA, and HA2, which contains the fusion peptide at its N-terminus. The fusion peptide is composed of an extremely conserved hydrophobic 50 | P a g e sequence. HA is the protypical member of the class I fusion protein family. When HA binds to the SA, the virion is internalized through endocytosis. In the endosomes the low pH, between 5 and 6, triggers fusion of the viral membrane and the endosomal membrane. The fusion is induced by a conformational change of HA. At the N-terminus, a long helix is formed as an unstructured linker, called a prehairpin, becomes helical. In this conformation the fusion peptide is launched into the target membrane and is subsequently embedded, connecting the viral and endosomal membranes. Figure 13: The life cycle of Influenza A. Influenza tethers its target cell through the binding of HA to SA. Subsequently the virus enters the Parallel the c-helix packed into the Nterminal trimeric coiled-coil grooves is cell via endocytosis. Inside the endosome, the low pH triggers a inverted forming a six-helix bundle. Due conformational change of HA that allows fusion of the endosomal and to the fusion peptide and the six-helix viral membranes releasing the RNA genome into the cytoplasm. The bundle, the two membranes are brought vRNPs are then translocated to the nucleus were transcription takes place. The negative sense RNA needs to be transcribed into a positive into close proximity greatly easing sense RNA in order to replicate the genome. The genomic RNA is merging (Belouzard et al. 2012). Apart packed together with NP and exported out of the nucleus. The from the conformational change, the assembly of the virion takes place at the plasma membrane where acidic environment also opens the M2 the viral membrane proteins concentrate. Finally the virion buds off. During this event, NA plays a key part cleaving SA and ensuring that the virus particle is released. ion channel. M2 exists in tetramers and forms a channel through which protons can enter. Activation of this channel Adapted from: http://nursingcrib.com/microbiology/influenza-viruslife-cycle/ results in an acidified viral core which permits the release of viral RNA packed in NP, called the viral ribonucleoproteins (vRNP). As mentioned before, vRNPs also contain the viral RNA polymerase complex. The transcription and translation subsequently occurs in the nucleus. In order to enter the nucleus, all the vRNP have a nuclear localization signal (NLS), although it is unknown whether the NLS is the key signal for the nuclear entry. Entry could additionally take place by binding importin α and β, which are also involved in nuclear import. As the influenza virus has a negative stranded RNA genome, it must first be transcribed into positive sense so it can be used for 51 | P a g e viral RNA production (Samji, 2009). For replication of the viral RNA, there is no need for a primer due to the fact that the viral RNA contains highly conserved untranslated regions (UTRs) on both the 3’ (12 nucleotides) as well as the 5’ site (13 nucelotides) (Dai et al. 2012). Furthermore, these UTRs are also partially inverse complementary so that they can base pair with each other forming corkscrewlike configurations. Influenza only encodes 11 proteins, as previously mentioned, hence has developed several ways to make use of the host machinery. For example cap snatching is observed during the replication/transcription process. Viral mRNAs contain a 5’ cap, however, there is no viral gene that encodes for this cap. The virus is able to use the host 5’ caps to protect its mRNAs from degradation. The cap is snatched by the viral protein PB2, which has an endonuclease activity, and cleaves the cap of the host mRNAs. Next the RNA needs to exit the nucleus, only negative sense vRNPs are exported from the nucleus. Once the vRNPs are exported out of the nucleus, the virions assemble and exit the cell. Viral assembly takes place at the plasma membrane as influenza is an enveloped virus. The virions exit the cell at the apical side through budding. The tail of M2 has been shown to be crucial for the virion formation. M1 also plays an important part in the budding off from the plasma membrane. Additionally, NA is needed to allow release of the novel virion from the plasma membrane since it is attached to SA. In particular NA is able to cleave off the SA hence removing them, which allows the release of the virus and the subsequent infection of adjacent cells (Figure 13) (Samji, 2009). 5.2 Influenza and autophagy The literature about the association between autophagy and influenza contains many discrepancies. While some studies state that autophagy is involved in replication, others conclude the contrary (Zhou et al. 2009; Gannagé et al. 2009). Not only the study of the virus replication and autophagy has produced contradictory results, determination whether the influenza virus is able to mediate autophagy maturation did so as well. While there is evidence for a maturation block by the virus, there is also proof that this block does not occur and that autophagy is fully functional (Gannagé et al. 2009; Law et al. 2010; Comber et al. 2011). Perhaps these differences are due to the fact that different cell types were used to study the influenza infection or simply because the different aspects of the interaction between autophagy and influenza infection were difficult to pinpoint. Since technology has greatly improved and the progression of autophagy can be tested in several ways, this latter point seems negligible. Taking into account that with HSV there is also discrepancy between different cell types, this seems the more likely explanation (Gobell et al. 2011). All the results and discrepancies will be reviewed in the upcoming sections. 52 | P a g e 5.2.1 Induction of autophagy Like most viruses influenza too induces autophagy upon entering the host cell, which has been shown by EM as well as by increased levels of LC3-II. Infection with influenza produces reactive oxygen species (ROS) via the nox2 NADPH oxidases. These ROS damage Atg4 leading to the accumulation of LC3-II, which results in autophagosome formation and accumulation. The influenza virus has moreover been shown to upregulate the autophagy proteins Atg7, Atg5 and Atg12 (Dumit et al. 2012). If the ROS are eliminated for example using the antioxidant procyanidin, autophagosome formation is decreased. This same component has been shown to decrease the expression of the overexpressed Atg7, Atg5 and Atg12. This suggests that both the ROS and overexpression of the Atg proteins are indeed partially responsible for the autophagosome formation (Dai et al. 2012). The induction of autophagy in MDCK and A549 cells infected with H1N1 (A/WSN/33) is initiated at least via the mTOR pathway as phosphorylation of p70S6K, a downstream effector of this kinase, decreases upon infection indicating that mTOR is inhibited, something that induces autophagy (Zhou et al. 2009). Surprisingly, however, A549 cells infected with the A/newcoledonia/20/1999 H1N1 strain do not seem to inhibit mTOR (Sun et al. 2012). This suggests that autophagy might also be regulated by another pathway and that this might be strain-related. Although autophagy is induced during the influenza virus infection, it does not contribute to antigen presentation as seen during other viral infections. CD4+ T cell responses were analyzed in Atg7 knockout cells and no difference was found in the responding CD4+ T cells compared to wt cells. Antigen presentation on MHCII was instead mediated by proteasome-dependent pathways (Comber et al. 2011). 5.2.2 Replication There is much debate on whether influenza needs autophagy for its replication. Inhibition of autophagy in MDCK and human lung cancer A59 cell lines infected with H1N1 (A/WSN/33) and H9N2 (A/chicken/Beijing/04), both using a chemical approach as well as RNA interference, resulted in a decrease in viral yield. This was however not due to inability to replicate the genome, as this happens in the nucleus, but was due to a decrease in the levels of M2, which is important in the assembly, entry and uncoating of the virus. Thus autophagy inhibition deregulates the viral protein turnover decreasing the yield. This suggests that autophagy, which should clear intracellular pathogens, is manipulated by influenza to deliver the right proteins for viral assembly (Zhou et al. 2009). Activation of autophagy is hence biphasic meaning that a part of an antiviral response is misused to support the viral replication (Ehrhardt et al. 2006). Additionally infecting autophagy deficient human mucoepidermoid pulmonary carcinoma cells with H1N1 (A/Puerto Rico/8/34) decreased the viral load. In this same paper cathepsin D was suggested to potentially be used by the influenza virus to corrupt the autophagic machinery during infection. Cathepsin D, which is involved in autophagy and apoptosis, is a cysteinyl-, aspartyl-, and serine-protease which is released from lysosomes upon 53 | P a g e activation. This was indicative as Pepstatin A, an inhibitor of cathepsin D, had a negtative effect on viral reproduction (Matarrese et al. 2011). Gannagé and colleagues contrastingly showed that infection of autophagy deficient mouse embryonic fibroblasts with H3N2 (A/Aichi/68) did not have an effect on the viral yield. In particular, they showed higher concentrations of RNA and viral proteins when autophagy was functional, however, this did not seem to affect the release of infectious virus (Gannagé et al. 2009). 4.2.3 To block or not to block A second main discrepancy in the field is whether autophagosomal maturation is blocked or not during an influenza infection. It has been shown that the influenza virus can prevent fusion of autophagosomes with lysosomes leading to an accumulation of autophagosomes. This phenomenon, however, does not occur until later during infection. This suggests that viral or perhaps even cellular factors need to be produced first before the fusion with the lysosome can be impaired. Several different strains, H3N2 (A/Aichi/68), H1N1 (A/WSN/33) and H1N1 (A/PR8/34), displayed this accumulation of autophagosomes. Transfection of M2 into the lung A59 cells is sufficient to cause this autophagosome accumulation phenotype (Gannagé et al. 2009). Indeed when excluding M2, by silencing M2 expression post infection, the autophagosomal accumulation phenotype was no longer observed. Unexpectedly, M2’s proton channel pump function did not contribute to the block of fusion with the lysosomes. Instead M2 co-immunoprecitpitated with Beclin 1 (Gannagé et al. 2010). Beclin 1 is a protein frequently targeted by viral proteins. In complex with UVRAG, it is important in the maturation of autophagosomes. Interaction with Beclin 1 at this time point could thus indeed result in a block of the late steps of autophagy (Munz, 2011). The exact interaction between Beclin 1 and M2 is not yet unraveled. Protein-protein interactions and dynamics will have to be further elucidated to determine the exact mechanism via which M2 interacts with Beclin 1 and if this is done in the context of the UVRAG-containing complex. Cells deficient for autophagy have increased susceptibility to apoptosis. The fact that the survival mechanism, autophagy, is blocked late during infection could indicate that the block of autophagy might be set in motion by the virus after it has successfully replicated. The exact benefit for the influenza virus to induce cell death by inhibiting autophagy remains unclear. This benefit might lie in depleting immune cells thus reducing the immune response. Macrophages are very sensitive to influenza virus-induced cell death (Bender et al. 1998). Lung epithelial cells are as well and consequently this could serve to lift the lung barrier allowing influenza to infect deeper into the lung tissue (Baskin et al. 2009). Apoptosis additionally limits the pro-inflammatory cytokine response. Furthermore the antigens are trapped in the autophagosomes and as a result they cannot be presented on the MHC complexes on the cell surface. All together, these observation support a 54 | P a g e model where the virus induces cell death via apoptosis and it combines it with a block of autophagy to possibly accelerate apoptosis of the infected cells with minimal immunogenicity (Gannagé et al. 2009). Nonetheless there are other reports where this block of autophagy was not detected. By both assessing LC3 as well as p62 degradation, two separate studies revealed induction of functional autophagy post infection. This was done in several cell lines including fibroblasts and primary DCs (Law et al. 2010; Comber et al. 2011). This, hence, again raises the possibility that perhaps the influenza virus life cycle is different depending on which strain as well as which cell type is used for the experiments. 5.2.4 H5N1-induced autophagy-mediated cell death The avian influenza virus H5N1 is, as mentioned above, highly pathogenic having a mortality rate of 50%. In embryonic mouse fibroblasts cell death was caused by influenza infection via autophagy. Autophagy is mostly a survival mechanism but when there is too much autophagy in the cell, this can contribute to the induction of cell death. H5N1 triggers this type of autophagy by inhibiting mTOR. Accordingly, cells with hyperactive mTOR were not susceptible to H5N1. The exact mechanism of how H5N1 inhibits mTOR is currently unknown, but there are indications that it is through the activation of the TSC1/2 protein complex. Furthermore the poor cell viability caused by H5N1 infection could be rescued by treating the cells with an autophagy inhibitor. These results were obtained using a virus that was inactivated suggesting that autophagy contributes to the pathogenicity independently from viral replication (Ma et al. 2011). The HA protein of H5N1 was shown to be potentially involved in inducing cell death via autophagy (Sun et al. 2012). If these results stand, autophagy inhibitors could be useful to diminish the tissue damage caused by H5N1. 5.3 Future directions Clarity about the exact role of autophagy during influenza virus infection has as not been provided yet. A substantial amount of contradicting literature has been published over the years suggesting that the difference in influenza virus strain as well as the difference in infected cells might have a large influence on the way autophagy is manipulated by the virus (Figure 14) The only result that is common in all the works is that influenza virus infection induces autophagy. One of the mechanisms that was proposed was the inhibition of mTOR. Nonetheless H1N1, which inhibits mTOR during the 24 hours post infection, has also been shown to induce autophagy independent of mTOR 36 hours post infection (Zhou et al. 2009; Sun et al. 2012). Two different strains (A/WSN/33 and A/newcoledonia/20/1999 respectively) were used during these experiments. This difference along with the different time post infection might have influenced the preferred pathway via which autophagy was induced. It would be interesting to elucidate the pathway used by the second H1N1 strain to trigger autophagy and see whether mTOR is crucial at an earlier time point or not. It seems 55 | P a g e peculiar that the main mechanism to induce autophagy is not important 36 hours post infection. Perhaps the virus somehow induces excessive ER stress and hence causes autophagy via this way. Further investigation should shed more light on the different pathways via which autophagy can be induced by influenza. The next step in the viral life cycle, replication, and the role of autophagy during this process is under debate. Autophagy has been indicated to be necessary for replication as well as be dispensable for this step of the virus infection. As mentioned in the replication section, the strains that have led to these varying conclusions were different. Two different H1N1 strains and a H9N2 strain were shown to decrease replication when autophagy was blocked while a H3N2 strain showed the opposite (Zhou et al. 2009; Gannagé et al. 2009; Matarrese et al. 2011). This difference might partially account for the different outcome. Furthermore the cells infected were distinct as well. MDCK, human lung cancer A59 and mucoepidermoid pulmanry carcinoma cells infected with the influenza virus showed an autophagy-dependent viral replication while mouse embryonic fibroblasts displayed an autophagy-independent replication. Thus, autophagy dependent replication was seen in epithelial cells which are the primary cells infected with the influenza virus (Zhou et al. 2009; Gannagé et al. 2009; Mataresse et al. 2011). Yet another difference was the methods via which viral yield was measured. Gannagé and colleagues made use of an MDCK plaque assay while Zhou and Matarrese resolved the viral yield using an HA assay. These different assays might also contribute to the contradicting results as the two papers using the same assay both showed an autophagy-dependent replication. It is key to approach the issue of autophagy and influenza virus replication by using the same tools and techniques in order to establish firm conclusions. At a later time point of the influenza virus infection, there is yet another discrepancy; whether autophagy is blocked or remains functional throughout the whole infection. M2 was shown to be the viral protein responsible for blocking autophagosome fusion with lysosomes in A59 cells. This block in autophagy was observed with two different H1N1 strains as well as the H3N2 strain. M2 is able to bind the subpopulation of Beclin 1 possibly in complex with UVRAG, which blocks autophagosome maturation. The exact interaction between M2 and Beclin 1 has not been elucidated. It is know that the binding site on M2 lies somewhere in the first 60 amino acids either at the ectodomain (1-24) or at the cytoplasmic amphipatic helix (46-62) (Gannagé et al. 2009; Munz. 2011; Dumit et al. 2012). Looking into the molecular details of this protein-protein interaction, perhaps using crystallography, is an elegant next step, which would shed more light on the exact involvement of M2 in autophagy. Using the same techniques, p62 accumulation and GFP-LC3, autophagy was shown to progress normally in fibroblast infected with H1N1 (PR8), the same strain used in by Gannagé and coworkers (Comber et al. 2011). Primary DCs also showed normal autophagy in cells infected with two different 56 | P a g e Figure 14: Autophagy and Influenza infection. The interaction of the influenza virus with autophagy is surrounded by many discrepancies. Autophagy is induced upon entry of influenza virus into the cell most likely via the mTOR pathway. This has been shown for both H5N1 and H1N1 strains but was also disproved for H1N1. Two different H1N1 strains were used so perhaps this might explain the observed difference. Subsequently different reports have suggested that autophagy is necessary for the replication while others have shown that autophagy is indispensible for viral replication. It is thought that autophagy induction during early infection leads to cell survival allowing the virus to replicate. Nonetheless during late infection, when the virus has successfully replicated, it causes apoptosis of the host cell. This is done via pro-apoptotic proteins (that have not been discussed within this chapter) but also through autophagy. During the H5N1 avian influenza, the increased in autophagy has been shown to be the cause for the induction of apoptosis. There are also some reports, however, which indicate that autophagy is blocked through the inhibition of the fusion between autophagosomes and lysosomes by the viral protein M2. M2 interacts with Beclin 1 and UVRAG thus blocking autophagosome maturation. This accumulation of autophagosomes eventually leads to cell death. All question marks indicate processes with contradicting results described in the literature H1N1 strains and H9N2 (Law et al. 2010). In these three papers, autophagy was hence assessed by the same techniques consequently the technique employed for the experiments is most likely not the reason of the observed differences. Perhaps the sole difference of cell type used for the experiment could explain the different, something that is not unprecedented because this was also shown for the herpesviruses. To my best knowledge, there are no reports which use different strains to infect the same cell type and consequently it is difficult to determine whether the strain could also have an effect on the manipulation of autophagy in a specific cell type, though it seems a likely scenario. 57 | P a g e Influenza virus infection eventually leads to a high susceptibility of the host cell to death as is seen both in H5N1 infection, through the induction of autophagy, and in A59 cells infected with H1N1 or H3N2, via a block of autophagy (Gannagé et al. 2009; Ma et al. 2011). Autophagy is a double-edged sword, that is a survival mechanism in normal conditions but when it is excessively induced or manipulated by the virus, it can contribute to the induction of cell death. Influenza virus induces autophagy upon infection, which is most likely extending the life of the host cell so that the virus can efficiently replicate (Gannagé et al. 2009). Additionally autophagy has been shown not to contribute to antigen presentation during influenza virus infection, which reduces the need for the virus to inhibit autophagy at an early time point (Comber et al. 2011). Inhibition of autophagy during the H5N1 infections was suggested to limit the pathology of this strain (Ma et al. 2009; Sun et al. 2012). The use of this approach as a therapy, however, needs a lot more research considering the fact that inhibiting autophagy in all tissues might lead to many toxic side effects. Additionally, as was shown by Gannagé and colleagues, inhibition of autophagy raises sensitivity to apoptosis so it might not be so beneficial for fighting this avian influenza virus. Nonetheless as this infection has a mortality rate of 50%, perhaps the side effects will not be as severe as the potential outcome of the disease and hence might be useful anyway. In conclusion, there is a substantial amount of discrepancies concerning the interaction between autophagy and the influenza virus infection. The exact role of autophagy will need to be elucidated both in the process of viral replication as well as in the maturation of autophagosomes occurs during the infection (Figure 14). These investigations will most likely need to be done in a strain and/or cell type specific setting as there is clear indication that divergence of strain and cell type might be the cause of certain discrepancies which exist in the field. Furthermore it is advised to make use of the same tools and techniques when analyzing certain aspects of the infection. 58 | P a g e 6. Conclusions In this thesis herpes, vaccinia, corona and influenza viruses have been discussed with regard to their interaction with autophagy. Between these four viruses, the ability of herpesviruses to manipulate autophagy has been most extensively studied. In contrast, there is no solid evidence linking CoV infection with autophagy. This latter virus, however, exploits LC3 for its replication thus indicating that autophagy proteins may also be involved in other processes (Reggiori et al. 2010). A recurring issue in the previous chapters was contradictory results. Viruses such as herpes and influenza displayed different effects on autophagy when different strains were used or when different types of cells were infected (Gannagé et al. 2009; Law et al. 2010; Comber et al. 2011). For example HSV blocked autophagy in fibroblasts but was unable to do this in DCs (Ramussen et al. 2011; Alexander et al. 2007). Furthermore, although it seems beneficial for a virus to inhibit autophagy and interfere with its potential destruction as well as with the presentation of epitopes on the cell surface recruiting the immune system, some viruses appear to induce autophagy. Even viruses from the same class such as the alphaherpesviruses behave differently. For example while HSV blocks autophagy, VZV induces it to expand the host cell’s life span (Cavignac et al. 2010). The four viruses are different both in biology as well as when it comes to their interaction with autophagy. Nonetheless, some do share similar characteristics regarding this interaction. The autophagy protein Beclin 1 is a very popular target when it comes to viruses. Inhibiting this protein abolishes the formation of autophagosomes. Many of the members of the different classes of herpes viruses contain a viral protein that targets Beclin 1. For HSV and CMV, ICP34.5 and TSR1, respectively, are responsible for binding Beclin 1 (Cavignac et al. 2010; Hakki et al. 2006). The gamma herpes viruses do not contain a homolog or ortholog of these proteins but encode v-Bcl-2 proteins. These proteins, which contain a BH3 domain, can bind Beclin 1 inhibiting autophagy (Cavignac et al. 2010; Sinha et al. 2008). Although the EBV v-Bcl-2 has not been experimentally proven to bind Beclin 1 and thus to be important in subverting autophagy, it seems likely that it could be functionally involved in this mechanism (Taylor et al. 2011). The gammaherpesviruses are not the only discussed viruses that code for v-Bcl-2 proteins; they are also found in vaccinia for example. Like EBV, however, these proteins have not been studied for their involvement in autophagy (Cooray et al. 2007; Grahan et al. 2008). Influenza on the other hand targets Beclin 1 via the proton channel protein M2. The exact interaction between these two proteins is, however, unknown (Gannagé et al. 2009). Altogether, three out of these four viruses analyzed in this report have developed different strategies 59 | P a g e as well as shared methods to target Beclin 1. This indicates that Beclin 1 is a very potent target for the inhibition of autophagy. Apart from Beclin 1, targeting the PKR system is another shared characteristic between these viruses. They all have been shown to inhibit this pathway, however, this has not always been linked with a negative regulation of autophagy (Orvedahl et al. 2008; Orvedahl et al. 2007; Cruz et al. 2011). Apart from initiating autophagy, PKR stimulates host shut off. The main reason for the viruses to target PKR might solely be to surpass the host shut off. This targeting could however also contribute to the inhibition of autophagy as was revealed for both the herpes and the vaccinia viruses (Alexander et al. 2007; Myskiw et al. 2011). Nonetheless the presence of additional ways to block autophagy such as the presence of ICP34.5’s BBD and the formation of the Atg12-Atg3 conjugate respectively suggest that the contribution via the block of PKR would be minimal. Autophagy is not only blocked by viruses, some viruses exploit the machinery for their own benefit. It is a survival mechanism and viruses could use this system to lengthen the host’ life span and thus allow replication to occur for a longer time. This was shown for VZV (Cavignac et al. 2010). Additionally in the latent stage of EBV, autophagy is induced by LMP1. This protein uses the mechanism to regulate its own levels. This process needs to be regulated tightly and a so far unidentified autophagy inhibitor, such as perhaps the v-Bcl-2 proteins, might play a role in this regulation (Lee et a., 2008). KSHV, another gammaherpesvirus, encodes the protein RTA that likewise induces autophagy via an unknown mechanism. This induction also needs to be tightly controlled and might be balanced by viral proteins such as v-Bcl-2 (Wen et al. 2010). This interplay with the induction of autophagy for the regulation of the latent stage is not seen in any of the herpes virus even though they all share the latent characteristic. This might be due to the fact that latency is its default stage for EBV and KSHV. Although there is no solid evidence linking CoVs to autophagy, transfection of cells with viral nsp6 did show induction of autophagy. Whether this induction is in fact also present during a CoV infection, is not known (Cottam et al. 2011). If this would indeed be the case the reasons behind inducing autophagy are speculative. Perhaps the virus, like VZV, benefits from lengthening the life span of the host. Another interesting speculation was suggested where immunomodulatory proteins would be targeted to the autophagosomes by the virus to decrease the immune response. This would suggest a somewhat chaperone-mediated system and would require the virus to avoid the autophagy system to prevent its own degradation. Nonetheless if the PKR antagonist encoded by CoV is able to overcome this induction, CoVs would fall in the category of the autophagy-blocking viruses. 60 | P a g e The mechanism interacting with autophagy, mainly the inhibitory ones, seem to have developed different molecules to target the same cellular protein. Nonetheless this does not hold true for all the viruses covered in this thesis. The vaccinia virus, although also containing PKR inhibitors and v-Bcl-2 proteins, displays a novel way to block autophagy, namely the formation of an Atg12-Atg3 conjugate (Moloughney et al. 2011). This has, to my knowledge, not been shown in any other virus but it remains to be seen whether it is typical only for vaccinia. The viral proteins involved in the formation of this conjugate have not been discovered yet and therefore it has not yet been possible to determine whether other viruses encode proteins with a similar structure and/or function. This could be the case since it is a very efficient system to inhibit autophagy hence perhaps other viruses have either dependently or independently developed the same/a similar system. In conclusion, the interaction with autophagy seems to be very important for numerous viruses. CoV is the only virus discussed in this thesis that has not been definitely linked to the modulation of autophagy. However there are some experimental indications that hint towards an involvement. This interaction will need to be further studied in order to truly say whether CoVs manipulate the autophagy system. The other three viruses all show clear links with autophagy, some stimulatory and others inhibitory. A substantial amount of information has been gathered regarding the interaction between these three viruses and autophagy. Nonetheless, in order to clarify the whole picture much more will need to be unveiled. Autophagy and viruses have an undeniable connection nonetheless many of the individual aspects are awaiting full discovery. 61 | P a g e References Alexander D. and D. Leib. 2008. Xenophagy in herpes simplex viru replication and pathogenesis. Autophagy. 4: 101-103 Alexander D., S. Ward et al. 2007. Analysis of the role of autophagy in replication of herpes simplex virus in cell culture. Journal of virology. 81: 12128-12134 Andrade R., M. Wessendarp et al. 2006. CD40 induces macrophage anti-toxoplasma gondii activity by triggering autophagy dependent fusion of pathogen containing vacuoles and lysosomes. J. Clin. Investigations, 116: 2366-2377 Baliji S., S. Cammer et al. 2009. Detection of nonstructural protein 6 in murine coronavirus infected cells and analysis of the transmembrane topology by using bioinformatics and molecular approaches. Journal of virology. 83: 6957-696283: Baskin C., H. Bielefeldt-Ohmann et al. Early and sustained innate immune response defines pathology and death in non human primates infected by highly pahtogenic influenza virus. PNAS. 106: 34553460 Belongia E. and A. Naleway. 2003. Smallpox vaccine: the good the bad and the ugly. Clinical medicine and research. 1: 87-92 Belouzard S., J. Millet et al. 2012. Mechanisms of coronavirus cell centry mediated by the viral spike protein. Virusses. 4: 1011-1033 Bender A., M. Albert et al. 1998. The distinctive features of influenza virus infection of dendritic cells. Immunobiology. 198: 552-567 Bengali Z., P. Satheshkumar et al. 2012. Orthopoxvirus species ans strain differences in cell entry. Virology. 433: 506-512 Bernasconi R. and M. Molinari et al. 2011. ERAD and ERAD tuning: disposal of cargo and of ERAD regulators from the mammalian ER. Current opinion in cell biology. 23: 167-183 Bernasconi R., J. Noack et al. 2012. Unconventional roles of nonlipidated LC3 in ERAD tuning and coronavirus infection. Autophagy. 8: 1534-1536 Boehme K., M. Guerrero. 2006. Human cytomegalovirus envelopen glycoproteins B and H are necessary for TRL 2 activation in permissive cells. The journal of immunology. 177: 7094-7102 Boni T., P. Cobey et al. 2006. Epidemic dynamics and antigenic evolution in a single season of influenza A. Porceedings of the royal society of biological sciences. 273:v1307-1316 Boscarino J., H. Logan et al. Envelope protein palmitoylaytions are crucial for murine coronavirus assembly. Journal of virology. 85: 289-2999 Brun J., D. Mahoney et al. 2012. Oncolytic vaccinia virus safely and effectively treats the skin tumors in mouse models of xeroderma pigmentosum. International journal of cancer. 0: 0-0 62 | P a g e Campadelli-Fiume G,, L. Menotti et al. 2012, Viral and cellular contributions to herpes simplex virus entry into the cell. Current opinion in virology. 2: 28-36 Cavignac Y. and A. Escaltine. 2010. Herpesviruses and autophagy: Catch me if you can! Virusses. 2: 314-333 Cavignac Y. and A. Escaltine. 2010. Herpesviruses and autophagy: Catch me if you can! Virusses. 2: 314-333 Chan G., M. Nogalski et al. 2009. Activation EGFR on monocytes is required for hcmv entry and mediated cellular motility. PNAS. 29: 22369-22374 Chaumorcel M., M. Lussignol et al. 2012. The human cytomegalovirus protein TSR1 inhibirs autophagy via its interaction with Beclin1. Journal of virology. 86: 2571-2584 Chaumorcel M., S. Sauquere et al. 2008. Human cytomegalovirus controls a new autophagydependent cellular antiviral defense mechanism. Autophagy. 4: 46-53 Clippinger AJ., T.G. Maguire et al. 2011. Human cytomegalovirus infection maintains mTOR activity and its perinuclear localization during amino acid deprivation. Journal of virology. 85:9369 –9376. Comber J., T. Robinson et al. 2011. Functional macorautophagy indcution by influenza A virus without a contribution to major histocompatibility complex class II restriction presentation. Journal of virology. 6453-6463 Connolly S., J. Jackson et al. 2011, Fusing structure and function: a structural view of the herpes virus entry machinery. Nature reviews Microbiology. 9:369-381 Cono J., C. Casey et al. 2003. Smallpox vaccination and adverse reactions. Guidance for clinicians. MMWR Recomm Rep. 52: 1-28 Cottam E., H. Maier et al. 2011. Coronavirus nsp6 proteins generate autophagosomes from the endoplasmic reticulum via an omegasome intermediate. Autophagy. 7: 1335-1347 Crotzer V. and J. Blum. 2010. Autophagy and adaptive immunity. Immunology. 131: 9-17 Cruz et al. 2009. Corona gene 7 counteracts host defenses and modulates virus virulence. Plos pathogens. 77-11-2012 e1002090 Dai J., G. Wang et al.2012. High throughput screening for anti influenza A virus drugs and study of the mechanism of procyanidin on influenza A virus induced autophagy. Journal of biomolecular screening, 17: 605-617 de Haan C. and F. Reggiori. 2008. Are nidoviruses hijacking the autophagy machinery. Autophagy. 4: 276-679 de Lima BD,, JS, May et al. 2005. Murine gammaherpesvirus 68 bcl-2 homologue contributes to latency establishment in vivo. Journal Gen Virology. 86: 31–40. Djengel J., O. Schoor et al. 2005. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. PNAS. 102: 7922-7927 63 | P a g e Dumit V. and J. Dengjel. 2012. Autophagosomal protein dynamics and influenza virus infection. Frontiers in immunology. 3: article 43 Egan D., J. Kim et al. 2011. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and Mtor. Autophagy. 7: 645-646 Ehrhardt C., H. Marjuki et al. 2006. Bivalent role of the phosphotidylinositol-3-kinase (PI3K) druing influenza virus infection and host cell defense. Cellular microbiology. 8: 1336-1348 Ekde N., S. Child et al. 2012. Poxvirus deploy genomic accordions to adapt rapidly against host antiviral defenses, Cell, 150: 831-841 Engleberg N., V. DiRita and T. Dermody. 2007. Schaechter's mechanisms of microbial disease. Fourth edition. Lippincott Williams & Wilkins English L., M. Chemali et al. 2009. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nature immunolgy. 10:480-489 English L., M. Chemali et al. 2009. Nuclear membrane derived autophagy, a novel process that participates in the presentation of endogenous viral antigens during HSV-1 infection. Autophagy. 5: 1026-1029 Enserink M. 2002. In search of a kinder, gentles vaccine. Science. 296: 1594 Esclatine A., M. Chaumorcel et al. 2009. Macroautophagy signaling and regulation. Current top microbiology and immunology. 335: 33-70 Feire A., H. Koss et al. 2004. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin like domain. PNAS. 101:15470-15475 Fliss P., T. Pechenick Jowers et al. 2012. Viral mediated redirection of NEMO/IKKgamma to autophagosomes curtails the inflammatory cascade. Plos pathogens. 8: e1002517 Gangappa S., LF. van Dyk et al. 2002. Identification of the in vivo role of a viral bcl-2. J Exp Med. 195: 931–940. Gannage M., D. Schmid et al. 2009. Matrix protein 2 of influenza A virus blocks autophagosome fusion with lysosomes. Cell host microbe. 6: 367-380 Gannage M., P. Ramer et al. 2010. Targeting Beclin 1 for viral subversion of macroautophagy. Autophagy. 6: 166-167 Gobell P. and D. Leib. 2012. Herpes simplex virus gamma34.5 interferes with autophagosome maturation and antigen presentation in dendritic cells. Mbio. 3(5): e00261-12 Goodrum F., K. Caviness et al. 2012. Human cytomegalovirus persistence. Cellular microbiology. 14: 644-655 Gu J., E. Gong et al. 2005. Mutliple organ infection and the pathogenesis of SARS. Journal of experimental medicine. 202: 415-424 64 | P a g e Gwinn D., D. Shackelford et al. 2008. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Molecular Cell. 30: 214-226 Haan C., M. Molinari et al, 2010. Autophagy independent LC3 funtion in vesicular traffic. Autophagy. 6: 994-996 Hailey D., A. Rambold et al. 2010. Mitochondria supply membranes for autophagsome biogenesis during starvation. Cell. 141: 656-667 Hakki M., E. Marshall EE et al. 2006. Binding and nuclear relocalization of protein kinase R by human cytomegalovirus TRS1. Journal of virology. 80:11817–11826. Haspot F., A. Lavault et al. 2012. Human cytomegalovirus entry into dendritic cells occurs via a macropinocytosis-like pathway in a pH independent and cholesterol dependent manner. Plos One. 7: E34795 Henderson D.A., B. Moss. 1999. Smallpox and vaccinia. Plotkin SA, Orenstein WA. Vaccines 3rd edition. Phiiladelphia, Pennsylvania: WB Saunders Hetz C. 2012. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nature molecular cell biology. 13:89-102 Isaacson M., A. Feire et al. 2007. Epidermal growth factor receptor is not required for hCMV entry or signaling. Journal of Virlogy. 81: 4241-4247 Israel A. 2010.The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol 2: a000158. Itzhaki R. and M. Wozniak. 2008. Herpes simplex virus type 1 in alzheimer's disease: the enemy within. Journal of Alzheimer's disease. 13: 393-405 Jacobs N., N. Bartlett et al. 2008,. Vaccinia virus lacking the bcl-2 like protein N1 induces a stronger natural killer cell response to infection. Journal of general virology. 89: 2877-2887 Kahn J., K. McIntosh et al. 2005. History and recent advances in coronavirus discovery. Pediatric Infect dis J. 24: 223-227 Kim L., J. Hayes et al. 2000, Molecular characterisation and pathogenesis of transmissible gastroenteritis coronavirus and procine respiratory coronavirus (PRCV) field isolates co-circulating in a swine herd. Arch Virology. 145: 1133-1147, Kirn D. and S. Thorne. 2009. Targeted and armed oncolytic poxvirus: a novel multi mechanistic therapeutic class for cancer. Nature reviews. 9: 64-71 Ku B., JS. Woo et al. (2008) Structural and biochemical bases for the inhibition of autophagy and apoptosis by viral BCL-2 of murine gammaherpesvirus 68. PLoS Pathogogens 4: e25 Law A., D. Lee et al. 2010. Cellular response to influenza virus infection: a potential role for autophagy in CXCL10 and interferon alpha induction. Cellular molecual immunology. 7: 263-270 65 | P a g e Lee D. and B. Sugden et al. 2008. The laten membrane protein 1 oncogene modifies B cell physiology by regulating autophagy. Oncogene. 1: 2833-2842 Lee. J. 2012. Vaccinia. Medscape reference: drugs, disease and procedures. http://emedicine.medscape.com/article/231773-clinical#a0218 Leib D., D. Alexander et al. 2009. Interaction of ICP34.5 with Beclin 1 modulates herpes simplex virus type 1 pathogenesis through the cotnrol of CD4+ T cell responses. Journal of virology. 83: 1216412171 Leidal A., D. Cur et al. 2012. Subversion of autophagy by Kaposi's sarcoma associated herpesvirus impairs oncogene- induced senesence. Cell host and microbe. 11: 167-180 Leung C., T. Haigh et al. 2010. Nuclear location of an endogenously expressed antigen, EBNA1, restricts acces to macroautophagy and the range of CD4 epitope display. PNAS. 107: 2165-2170 Levine B., N. Mizushima et al. 2011. Autophagy in immunity and inflammation. Nature. 469: 323-335 Liang C., J. Lee et al. 2008. Immune evasion in Kaposi's sarcoma associated herpes virus associated oncogenesis. Seminars in cancer biology. 18: 423-436 Lopper M. and T. Compton. 2004. Coiled coil domains in glycoprotein B a nd H are involved in CMV membrane fusion. Journal of virology. 8333-8341 Lussignol M., C. Queval et al. 2012. The herpes simplex virus type 1 Us11 protein inhibits autophagy through its interaction with the protein kinase PKR. Journal of virology. Epub 01158-12 Ma J., Q. Sun et al. 2011. Avian influenza A virus H5N1 causes autophagy mediated cell death through suppression of mTOR signaling. Journal of genetics and genomics. 38: 533-537 Matarrese P., L. Nencioni et al. 2011. Pepstatin A aters host cell autophagic machinery and leads to a decrease in influenza A virus production. Cellular physiology. 226: 3368-3377 McFarlane S., J. Aitken et al. 2011. Early induction of autophagy in human fibroblasts after infection with human cytomegalovirus or herpes simplex virus 1. Journal of virology. 85: 4212-4221 McLean J., E. Datan et al. 2009. Lack of Bax prevents influenza A virus induced apoptosis and causes diminished viral replication. Journal of virology. 83: 8233-8246 Mingo R., J. Han et al. 2012, Replication of herpes simplex virus: egress of progeny virus at specialised cell membrane sites. Journal of virology. 68:7084-77097 Moloughney J., C. Monken et al. 2011. Vaccinia virus leads to Atg12-Atg3 conjugation and deficiency in autophagosome formation. Autophagy. 7: 1434-1447 Montag C., J. Wagner et al. 2006. Human cytomegalovirus blocks tumor necrosis factor alpha- and interleukin-1beta-mediated NF-kappaB signaling. Journal of virology. 80: 11686–11698. Munz C. 2011 Beclin 1 targeting for viral immune escape. Viruses. 3: 1166-1178 66 | P a g e Murray P., K. Rosenthal and M. Pfaller, 2009, Medical Microbiology. Fifth edition. Elsevier Health Sciences Myskiw C., J. Arsenio et al. 2011. Comparative analysis of poxvirus orthologues of the vaccinia virus E3 protein- modulation of protein kinase r activity, cytokine responses and virus pathogenicity. Journal of virology. 85: 12280-12291 Nakagawa I., A. Amano et al. 2004. Autophagy defends cells against invading group A Streptococcus. Science. 360: 1037-1040 Orvedahl A. and B. Levine. 2008. Autophagy and viral neurovirulence. Cellular microbiology. 10: 1747-1756 Orvedahl A. and B. Levine. 2008. Viral evasion of autophagy. Autophagy. 4: 280-285 Orvedahl A., D. Alexander et al. 2007. HSV-1 ICP34.5 confers neurovirulence by targeting Beclin 1 autophagy protein. Cell host and microbe. 1: 23-35 Orvedahl A., S. MacPherson et al. 2010. Autophagy protects against Sindbis virus infection of the central nervous system. Cell host microbe. 7: 115-127 Osterholm M. 2005. Preparing the next pandemic. The new England journal of medicine. 352: 18391842 Palese P. and M. Shaw. 2007. Orthomyxoviridae: the viruses and their replication. In: Knipe, DM.: Howley PM., editors. Fields Virology. Philadelphia: Lippincott, Williams & Wilkins; 2007, Pattingre S., A. Tassa et al. 2005. Bcl-2 antiapoptotic protein inhibit Beclin 1 dependent autophagy. Cell. 122: 927-939 Pei Y., Z. Chen et al. 2011. Autophagy is involved in anti-viral activity of pentagalloylglucose (PGG) against herpes simplex virus type 1 infection in vitro. Biochemical and biophysical research communications. 405: 186-191 Perlman S. and J. Netland. 2009. Coronaviruses post SARS: Update on replication and pahtogenesis. Nature reviews microbiology. 7: 439-450 Peters G.A., D. Khoo et al. 2002. Inhibition of PACT‑mediated activation of PKR by the herpes simplex virus type 1 Us11 protein. Journal of virology. 76:11054‑11064. Pratt Z., J. Zhang et al. 2012. The latent membran portein 1 (LMP1) oncogene of Epstein-Barr virus can simultaneously induce and inhibit apoptosis in B cells. Journal of virology. 86: 4380-4393 Prentice E., W. Jerome et al. 2004. Cornavirus replication complex formation utilises components of cellular autophagy. The journal of biological chemistry. 279: 10136-10141 Pyo J., J. Na et al. 2012. Molecules and their functions in autophagy. Experimental and molecular medicine. 44: 73-80 67 | P a g e Radoshevich L., L. Murrow et al. 2010. ATG12 conjugation to ATG3 regulates mitochondrial homeostasis and cell death. Cell. 142: 590-600 Rasmussen S., K. Horan et al. 2011. Activation of autophagy by alphaherpesviruses in myeloid cells is mediated by cytoplasmic viral DNA through a mechanism dependen on stimulator of IFN genes. The journal of immunology. 187: 5264-5276 Reggiori F., C. de Haan et al.2011. Unconventional use of LC3 by coronaviruses through the alleges subversion of the ERAD tuning pathway. Viruses. 3: 1610-1623 Reggiori F., I. Monastryrska et al. 2010. Coronaviruses hijack the LC3-I positive EDEMosomes, ER derived vesicles exporting short lived ERAD reuglators for replication. Cell host and microbe. 7: 500508 Reinboth J., M. Ascierto et al. 2012. Correlates between host and viral transcriptional program associated with different oncolytic vaccinia virus isolates. Human gene therapy methods. 2:1-12 Roberts K. and G. Smith. 2008. Vaccinia virus morphogenesis and dissemination. Cell. 16: 472-479 Ruch T. and C. Machamer. 2012. The coronavirus E protein: assembly and beyond. Viruses. 4: 363382 Ryckman B., M. Chase et al. 2008, hCMV gh/gl/ul 128-131 interferes with virus entry into epithelial cells evidence for cell type specific receptors. PNAS. 16: 14118-14123 Saha A. and E. Robertson. 2011. Epstein-Barr virus-associated B cell lymphomas: Pathogenesis and clinical outcomes. Clinical cancer research. 17: 3056-3063. Saif L. 2010, Bovine respiratory coronavirus. Vet. Clin. North. Am. Food Anim. Pract. 26: 349-364 Samji T. 2009. Influenza A: understanding the viral life cycle. Yale journal of bio med. 82: 153-159 Schimdt F., C. Ernst et al. 2012. Poxvirus host cell entry. Current opinion in virology. 2: 20-27 Shi J. and H. Luo. 2012. Interplay between cellular autophagy machinery and postive stranded RNA viruses. Acta Biochim Biotphys Sin. 44: 375-384 Smith G., D. Viajakrishna et al.2009. Origins and evolutionary genomics of the 2009 swine origin H1N1 influenza A epidemic. Nature. 459: 1122-1125 Soohwan O., E. Xiaofei et al. 2010. Autophagy evasion in herpesviral latency. Autophagy. 6: 151-152 Soroceanu L., A. Akhavan et al. 2008. Platelet derived growth factor alpha receptor is required for hCMV infection. Nature. 18: 391-395 Speck S. and D. Ganem. 2010. Viral latency and its regulation: lessons from the gammaherpes viruses. Cell host microbe. 8: 100-115 Suarez A., R. Kong et al. 2011. Gammaherpesvirus 68 infection of endothelial cells requires both host autophagy genes and viral oncogenes for optimal survival and persistence. Journal of virology. 85: 6293-6308 68 | P a g e Sun S. and D. Wirtz, 2006. Mechanism of enveloped virus entry into host cells. Biophys J. 90: L10-L12 Sun Y., C. Li et al. 2012. Inhibition of autophagy ameliorates acute lung injury caused by avian influenza A H5N1 infection. Science signaling. 5: 212 ra16 Talloczy Z., H. Virgin et al. 2006. PKR-dependent autophagic degradation of herpes simplex cirus type 1. Autophagy. 2: 24-29 Taylor G., J. Mautner et al. 2011. Autophagy in herpesvirus immune control and immune escape. Herpesviridae. 2:2 Thor S., D. Hilt et al. 2011. Recombination in avian gamma coronavirus infectious bronchitis virus. Viruses. 3: 1777-1799, Tooze S. and T. Yoshimori. 2010. The origin of the autophagosomal memrbane. Nature cell biology. 12: 831-835 Tsuhako M., O. Augusto et al. Tempol ameliorates murin viral encephalomyelitis by preserving the blood brain barrier, reducing viral load, and lessening inflammation. Free rado Biol Med. 45: 704-712 Wah Wen K. and B. Damania. 2010. Kaposi sarcoma associated herpesvirus: molecular biology and oncogenesis. Cancer Letters 189: 140-150. Wang X., D. Huang et al. 2005. Integrin alphavbeta3 is a coreceptor for human cytomegalovirus infection. Nature medicine. 11:515-521 Wen H., Z. Yang et al. 2010. Enhancement of autophagy during lytic replication by Kaposi's sarcoma associated herpesvirus replication and transcription activator. Journal of virology. 84: 7448-7458 Wenhui L., J. Sui et al. The S protein of human coronavirus NL63 and the severe acute respiratory syndrome coronavirus bind overlapping regions of ACE2. Virology. 25: 367-374 Wileman T. 2006. Aggresomes and autophagy generate sites for virus replication. Science. 312: 875879 Xiaofei E., S. Hwang et al. 2009. Viral Bcl-2 mediated evasion of autophagy aids chronic infection of gammaherpesvirus68. Plos pahtogens. 5: e1000609 Yang Z. and D. Klionsky et al. 2010. Eaten alive: a history of macroautophagy. Nature cell biology. 12:9: 814-822 Zhang H., C. Monken et al. 2006. Cellular autophagy machinery is not required for vaccinia virus replication and maturation, Autophagy. 2: 91-95 Zhoa Z., L. Thackray et al. 2007. Coronavirus replication does not require the autophagy gene ATG5. Autophagy. 3: 581-585 Zhou Z., X. Jiang et al. 2009. Autophagy is involved in influenza A replication. Autophagy. 5: 321-328 69 | P a g e