This study guide was generated by ExamView Test Generator © Holt
advertisement

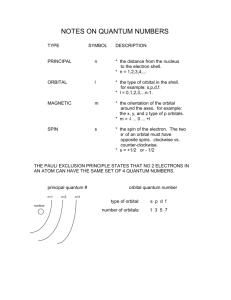
This study guide was generated by ExamView Test Generator © Holt 2009 and may not be duplicated without written permission of the publisher. Multiple Choice: 1. The product of the frequency and the wavelength of a wave equals the … a. # of waves passing a point in a second c. distance between wave crests b. speed of the wave d. time for 1 full wave to pass 2. Visible light, X rays, infrared radiation, & radio waves all have the same a. energy b. wavelength c. speed d. frequency 3. If electromagnetic radiation A has a lower frequency than electromagnetic radiation B, then compared to B, the wavelength of A is a. longer b. shorter d. exactly half the length of B’s wavelength c. equal 4. The wave model o flight does not explain a. light’s frequency b. the continuous spectrum c. interference d. the photoelectric effect 5. The emission of electrons from metals that have absorbed photons is called the _____ effect, a. interference b. photoelectric c. quantum d. dual 6. For an electron in an atom to change from the ground state to an excited state, a. energy must be released. c. radiation must be emitted. b. energy must be absorbed. d. the electron must transition from a higher to a lower energy level. 7. Bohr’s theory helped explain why a. electrons have a negative charge. b. most of the mass of the atom is in the nucleus. c. excited hydrogen gas gives off certain colors of light. d. atoms combine to form molecules. 8. According to Bohr, electrons cannot reside at _____ in the figure below. a. point A b. point B c. point C d. point D B nucleus D A C 9. Which model of the atom explains the orbitals of electrons as waves? a. the Bohr model b. the quantum model c. the Rutherford model d. Planck’s theory 10. All the following describe the Heisenberg uncertainty principle except a. it states that it is impossible to determine simultaneously both the position & velocity of an electron or any other particle. b. it is one of the fundamental principles of our present understanding of light and matter. c. it helped lay the foundation for the modern quantum theory. d. it helps to locate an electron in an atom. 11. The ______ quantum number indicates the position of an orbital about the 3 axes in space. a. principal b. angular momentum c. magnetic d. spin 12. The main energy levels of an atom are indicated by the _______ quantum numbers. a. orbital b. magnetic c. spin d. principal 13. The angular momentum quantum number indicates the a. orientation of an orbital around the nucleus c. direction nof the spin of the electron in its orbital b. shape of the orbital d. main energy level of an orbital 14. The number of sublevels within each energy level of an atom is equal to the value of the __ quantum number. a. principal b. angular momentum c. magnetic d. spin 15. The spin quantum number indicates that the number of possible spin states for an electron in an orbital is a. 1 b. 2 c. 3 d. 5 16. The set of orbitals that are dumbbell shaped and directed along the x, y, & z axes are called ____ orbitals. a. d b. p c. f d. s 17. A spherical electron cloud surrounding an atomic nucleus would best represent _____ orbital(s). a. an s b. a px c. combination of px & py d. combination of s & px 18. The major difference between a 1s & a 2s orbital is that a. the 2s orbital can hold more electrons. c. the 2s orbital is at a higher energy level. b. the 2s orbital has a slightly different shape d. the 1s orbital can have only one electron. 19. An orbital that can never exist according to the quantum description of the atom is a. 3d. b. 6d. c. 8s d. 3f 20. The letter designations for the 1st 4 sublevels with the maximum # of electrons in that energy level is a. s:2, p:4, d:6, f: 8 b. s:1, p:3, d:5, f: 7 c. s:2, p:6, d:10, f: 14 d. s:1, p:2, d:3, f: 4 c. 5 d. 7 c. 9 d. 18 21. The number of orbitals for the d sublevel is a. 1 b. 3 22. The number of orbitals for the f sublevel is a. 5 b. 7 23. The total number of orbitals that can exist at the second main energy level is a. 2 b. 3 c. 4 d. 8 24. How many electrons can occupy the s orbital at each energy level? a. 2, if have opposite spins b. 2, if have the same spin c. one d. no more than 8 25. If n is the principal quantum number of a main energy level, the number of electrons in that energy level is a. n b. 2n c. n2 d. 2n2 26. One main energy level can hold 18 electrons. What is n? a. +1/2 b. 3 c. 6 d. 18 27. If 8 electrons completely fill a main energy level, what is n? a. 2 b. 4 c. 8 d. 32 28. If the third main energy level contains 15 electrons, how many more could it possibly hold? a. 0 b. 1 c. 3 d. 17 29. A single orbital in the 3d level can hold ___ electrons. a. 10 b. 2 c. 3 d. 6 30. The statement that an electron occupies the lowest available energy orbital is a. Hund’s rule. c. Bohr’s law. b. the Aufbau principle. d. the Pauli exclusion principle. 31. “Orbitals of equal energy are each occupied by 1 electron before any is occupied by a 2nd electron, and all electrons in singly occupied orbitals must have the same spin” is a statement of a. the Pauli exclusion principle b. the Aufbau principle c. the quantum effect d. Hund’s rule 32. The statement that no 2 electrons in the same atom can have the same 4 quantum numbers is b. Hund’s rule a. the Pauli exclusion principle c. Bohr’s law d. the Aufbau principle 33. Two electrons in the 1s orbital must have different spin quantum numbers to satisfy the a. quantum rule b. magnetic rule c. Pauli exclusion principle d. Aufbau principle 34. The sequence in which energy sublevels are filled is specified by the a. Pauli exclusion principle c. Lyman’s series b. orbital rule d. Aufbau principle 35. The Aufbau principle states that an electron a. can have only 1 spin number c. must be paired with another electron b. occupies the lowest available energy level d. must enter an s orbital 36. In the electron configuration for scandium (4521Sc), what is the notation for the 3 highest-energy electrons? a. 3d1 4s2 b. 4s3 c. 3d3 d. 4s24p1 37. Which of the following lists atomic orbitals in the correct order they are filled according to Aufbau principle? a. 1s2s2p3s4s3p3d4p5s c. 1s2s2p3s3p4s4p3d4d b. 1s2s2p3s3p4s3d4p5s d. 1s2s2p3s3p3d4s4p5s 38. Both 6429Cu & 5224Cr appear to break the pattern in the order of filling the 3s & 4s orbitals. This change in order is expressed by a. an increase in the # of electrons in both the 3d & 4s orbitals. b. a reduction in the # of electrons in both the 3d & 4s orbitals. c. a reduction in the # of electrons in the 3d orbital & an increase in the 4s orbital. d. a reduction in the # of electrons in the 4s orbital & an increase in the 3d orbital. 39. The element with the electron configuration 1s22s22p63s23p2 is a. 2412 Mg b. 126C c. 3216S d. 2814Si 40. The electron notation for 2713Al is a. 1s22s22p33s23p33d1 b. 1s22s22p63s22d1 c. 1s22s22p63s23p1 d. 1s22s22p9