Extraction of Metals (N4&5) – Homework Questions
advertisement
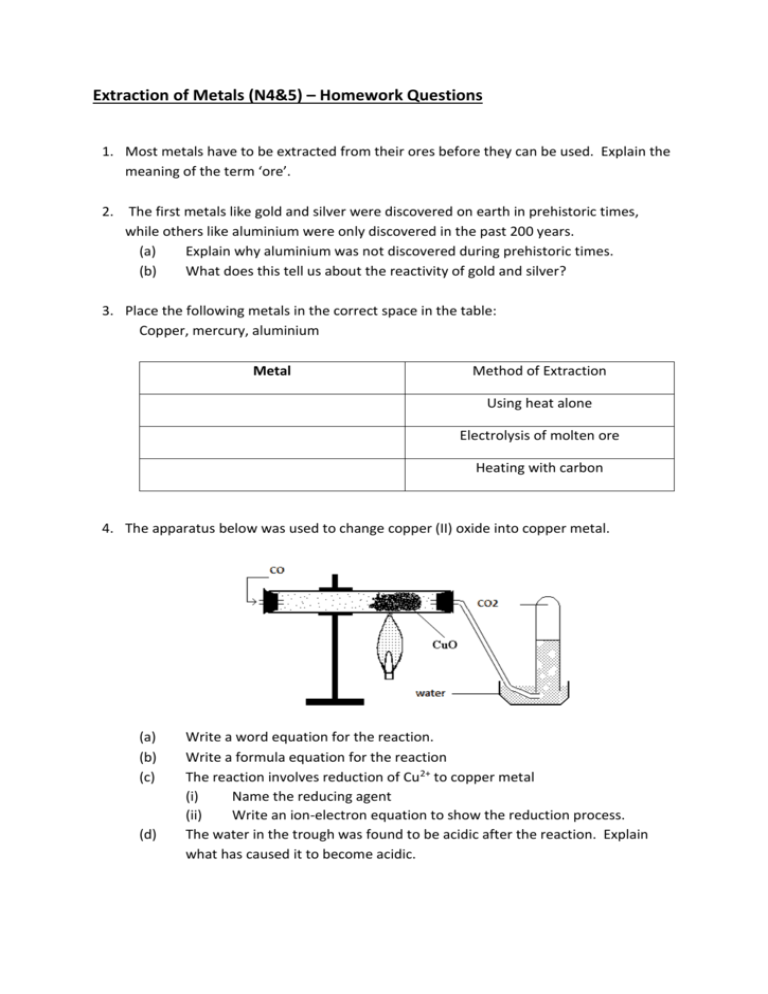
Extraction of Metals (N4&5) – Homework Questions 1. Most metals have to be extracted from their ores before they can be used. Explain the meaning of the term ‘ore’. 2. The first metals like gold and silver were discovered on earth in prehistoric times, while others like aluminium were only discovered in the past 200 years. (a) Explain why aluminium was not discovered during prehistoric times. (b) What does this tell us about the reactivity of gold and silver? 3. Place the following metals in the correct space in the table: Copper, mercury, aluminium Metal Method of Extraction Using heat alone Electrolysis of molten ore Heating with carbon 4. The apparatus below was used to change copper (II) oxide into copper metal. (a) (b) (c) (d) Write a word equation for the reaction. Write a formula equation for the reaction The reaction involves reduction of Cu2+ to copper metal (i) Name the reducing agent (ii) Write an ion-electron equation to show the reduction process. The water in the trough was found to be acidic after the reaction. Explain what has caused it to become acidic. 5. Julia was carrying out experiments on lithium oxide, silver oxide and tin oxide. The following results were obtained. Metal Oxide Heat Alone Heat with Carbon A No reaction No reaction B metal and oxygen produced metal and oxygen produced C No reaction Metal produced (a) (b) (c) Name the metal oxides A, B and C Why did metal oxide A have no reaction in either experiment? How could you extract metal A from its metal oxide? 6. When a metal is extracted from its ore, metal ions are changed to metal atoms. Name this type of chemical reaction. 7. Calculate the percentage weight of sulphur in sulphur dioxide 8. Calculate the weight of hydrogen in hexane (C6H12). 9. What is the percentage mass of calcium in chalk (CaCO3)? 10. Calculate the percentage of oxygen in Aluminium hydroxide.











