Monitoring of anti Xa activity in patients undergoing high volume
advertisement

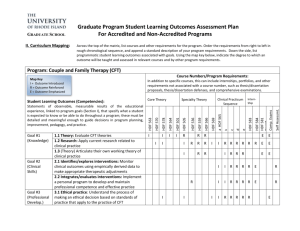
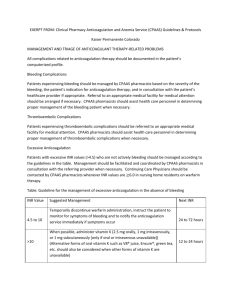
P155 MONITORING OF ANTI XA ACTIVITY IN PATIENTS UNDERGOING HIGH VOLUME HAEMODIAFILTRATION (HDF) USING TINZAPARIN FOR ANTICOAGULATION Raza H.1, Large S.2, Odgers E.2, Schulz M.1 ¹Renal Unit Royal Liverpool University Hospital, ²St Helens & Knowsley Dialysis Unit Introduction: Unfractionated heparin has been traditionally used for anticoagulation during haemodialysis. Following the 2002 European Best Practice Guideline recommendation lowmolecular-weight heparin (LMWH)is increasingly being used as an alternative across the United Kingdom, though without good quality evidence supporting this. Anticoagulation on haemodialysis using low-molecular-weight heparin has proven to be an effective, well tolerated and convenient alternative to unfractionated heparin. However there are little data for LMWH in high volume HDF and consequently Tinzaparin (INNOHEPR) is not licensed for this dialysis mode. Theoretically it can be assumed that the pharmacokinetics of LMWH are affected by the increased convective clearance of middle molecules compared to conventional HD. We conductedthese audits to monitor the safety of our local clinical protocol advocating the use of Tinzaparin. Methods: In order to establish the safety of our local HD anticoagulation protocol for HDF we were monitoring periodically anti-Xa levels in a prospective audit cycle in 3-6 monthly intervals. Data was collected from a single peripheral dialysis Unit. All patients were above 18 years of age, established on 4hrs high volume HDF via AVF or Tunnelled neck line using a high-flux polysulfone membranes (Fresenius Medical Care).Anti-Xa levels were checked 2hrs. into dialysis and after 4 hrs.(end of HD).BP, pump speed, events of bleeding, clotting and hypotension, venous chamber clotting scale, post HD bleeding(vascular access compression time -arterial and venous) and Tinzaparin dose were recorded. Results: Initial Pilot audit: the 4 hour ani-Xa Level ranged between 0.00-0.27 U/ml. First audit (21 patients):2 hr. anti Xa level range between 0.00-0.88 U/ml after, 4hr. anti-Xa level were not detectable. No major bleeding or hypotension episodes occurred /Venous chamber clotting scale 1(12) 2(6) 3(4) 4(1)/Vascular access pressure Arterial (2-25 min) Venous(3-20min) 1 (clotted circuit and lost pack)Second audit (6 month later 15 patients): 2 hrs. anti-Xa level range between0.00-0.51 U/ml after ,4 hr. anti-Xa level ranged 0.00-0.17 U/ml. No major bleeding, clotting or hypotension episodes occurred. In both audits venous chamber clotting scale and post HDF bleeding time (measured as pressure applied in minutes) did not correlate with anti-Xa activity measured. Conclusion: • Our simple, three fixed dose protocol for anticoagulation with Tinzaparin in HDF guided by clinical observation and events is practicable and safe. • In the absence of renal elimination in most patients anticoagulation as measured using anti-Xa activity shows a consistent drop toward the end of Haemodiafiltration • In conventional HD it has been previously shown that anti-Xa level decrease at the end of HD to a level that is considered safe. Our results suggest possibly even further reduction of anti-Xa activity at 2hrs and 4hrs potentially achieved by increased middle molecule clearance in high volume HDF • Applying HD protocol for anticoagulation with Tinzaprin to high volume HDF might potentially cause “underdosing” • Interestingly our observation seem to suggest clinical events such as post HD clinical bleeding time or venous clotting scale seem to be unrelated to measured anti Xa activity this suggests in our view that anti-Xa activityis an unreliable marker for effect of anticoagulation in HDF patients • The pharmacokinetic of LMWH in high volume HDF needs further research