Anticoag with Heparin
advertisement

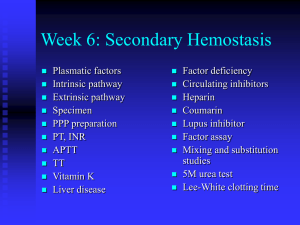
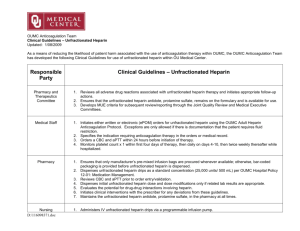
Heparin in CRRT Benan Bayrakci, 2014 McLean Heparin Binds to Antithrombin III and Speeds up its action by a 1000-fold Antitrombin 3 Inactive Thrombin (IIa) V, VIII, XIII, Fibrinogen Antitrombin 3 Inactive Factor Xa Common Pathway Antitrombin 3 Inactive Factor IXa, XIIa Contact activation Pathway Also • Releases Tissue factor pathway inhibitor form endothelium • Binds to platelets and inhibits platelet aggregation UFH is made up of heparin molecules of varied sizes (5–30 kDa). Larger fragments: Anti-IIa activity and are cleared rapidly (measured by APTT) Smaller fragments: Inhibit Xa (normal APTT because of its delayed clearance) Warkentin, 2003 Warkentin, 2003 Protocols • • • • • • Heparin infusion prior to filter Bolus with 10-30 units/kg Infuse heparin at 5-20 units/kg/hr Post filter ACT measurement Adjust post filter ACT 180-200 secs Interval of checking is local standard and varies from 1-4 hr increments Monitoring • • • • Anticoagulant effect Filter efficacy Circuit life Complications • Efficacy of UFH for prolonging filter life is proportional to the APTT and not to the heparin dose • APTT is maintained between 34–45 seconds, or an APTT of 1.5–2.0 times normal Advantages • • • • • • • Most commonly used anticoagulant worldwide for CRRT Widely available Simple to monitor Inexpensive Familiar to physicians Easy to administer Reversible with protamine Disadvantages • Unpredictable and complex pharmacokinetics resulting in dosing variability • Heparin-induced thrombocytopenia • Heparin resistance because of low patient antithrombin levels • Risks of hemorrhage (bleeding episodes: 10–50%, mortality 15%) Regional Unfractionated Heparin–Protamine • Anticoagulant effects are restricted to the circuit, lower risk of bleeding • Difficulty in estimating the amount of protamine required • Initial ratio of 100 between prefilter heparin (in units) and postfilter protamine (in mg) • Subsequent adjustment according to APTT • Requires measurement of both circuit and patient APTT • The heparin–protamine complex is taken up by the reticuloendothelial system and broken down, then heparin and protamine are released back into the circulation • Protocols are cumbersome and difficult to standardize • Protamine infusion is associated with hypotension, anaphylaxis, cardiac depression, leukopenia, and thrombocytopenia Low Molecular Weight Heparins • Have higher anti-Xa • Pharmacokinetics is more predictable because of less plasma protein binding • More reliable anticoagulant response • Lower incidence of HIT • Reversal with protamine is less effective • Dalteparin, enoxaprin, and nadroparin have been studied in CRRT • Excreted renally, their effects are prolonged in renal failure • Special coagulation assays are required to monitor anti-Xa activity Longer filter survival times, and lower cost, bleeding complications not increased Determination of anti-Xa levels aiming at 0.25–0.35 IU/ml is recommended 174 patients No significant difference in survival up to day 30 There were more metabolic disturbances with citrate anticoagulation Regional anticoagulation with citrate does not eliminate any need for heparin since, many other indications for systemic anticoagulation may emerge during therapy Citrate anticoagulation has distinct advantages with regard to haemofilter patency and the risk of HIT and bleeding Neither citrate nor heparin anticoagulation should be regarded as a therapeutic standard, since there is no advantage of one of these substances with regard to patient mortality. For patients who are at low risk of bleeding and do not have other contraindications to heparin such as HIT, consider using UFH For patients who are at high risk for bleeding and who do not have liver failure, consider using regional citrate anticoagulation The ideal anticoagulant should provide • Optimal anti-thrombotic activity • Minimal bleeding complications • Negligible systemic effects • Inexpensive • Have a short half-life • Can be easily reversed • Monitoring methods should be simple and available Heparin was used to be manufactured in factories from porcine intestine or bovine lung