Mechanical Circulatory Support Program
advertisement

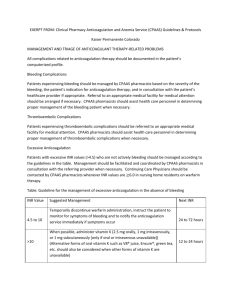
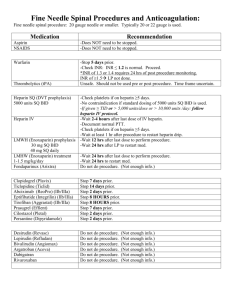
Vanderbilt Heart and Vascular Institute Mechanical Circulatory Support Program Anticoagulation and Antiplatelet Protocol for HeartMate II Purpose: Combined anti-coagulation therapy with anti-platelet therapy is provided to prevent thromboemboic events at the same time preventing hemorrhage that are risk factors associated with patients supported by mechanical circulatory support devices. Considerations: Following discharge, anticoagulation will to be managed by VUMC’s Anticoagulation Clinic. There are reports describing increased platelet aggregation and endothelial activation in the setting of infection, particularly bacteremia, resulting in increased risk of stroke. In this setting, antiplatelet therapy may need to be intensified. HEARTMATE II LVAD Anticoagulation reversed prior to leaving operating room POD 1: Begin ASA 81mg daily POD 3-5: Begin warfarin using the following guidelines: o Target INR 2.0-2.5 unless there is another indication for anticoagulation. Then, standard guidelines apply: AF or history of recurrent DVT/PE: INR 2.0-3.0 Mechanical mitral valve: INR 2.5-3.5 o In general, heparin will NOT be used to bridge patients in the postoperative period unless there is an indication to do so (i.e. mechanical valve). o If any Heartmate II patient is on a Heparin drip on a Step-down unit it will be physician managed only and these patients will not receive a bolus. Longitudinal care: In general patients will be maintained on: o ASA 81mg daily o Warfarin with goal INR as outlined above o In select patients, Plavix 75mg daily may be used if pump thrombus is suspected. Indications for Lovenox/Fondaparinux bridging Post-Discharge: o Bridging with Lovenox (1mg/kg/dose bid) as an outpatient is indicated in the following situations: INR < 1.7 for all patients INR < 2.2 if mechanical mitral valve o For patients with HIT, weight based fondaparinux will be used for the above indications. Appropriate weight base dosing: < 50kg: 5mg daily 50-100kg: 7.5mg daily > 100kg: 10mg daily o Lovenox should be renally dosed. Both Lovenox and fondaparinux should be used with caution in patients with renal insufficiency. In patients with severe renal insufficiency (GFR < 30), Lovenox and fondaparinux should not be used, and patients should be admitted for IV anticoagulation if bridging is indicated (see criteria above). Indications for admission for subtherapeutic INR: o Suspected pump thrombosis o LMWH or Fondaparinux is contraindicated Renal insufficiency with GFR < 30 IV Heparin (or Argatroban if HIT) will be used with goal PTT 50-70 Right heart catheterizations o Can be done by Dr. Zhao without holding Coumadin if INR < 2.5. INR needs to be checked 2 days prior to procedure to make sure it is not supratherapeutic. o If Dr. Zhao is not available for the procedure, Coumadin should be held, and patients should be bridged with Lovenox/fondaparinux as described above. Revised 2/16/12 Approved By: Simon Maltais, MD, PhD, FRCSC Mary E. Keebler, MD Surgical Director Medical Director Heart Transplant and Circulatory Support Devices Circulatory Support Devices