Respiratory Physiology
advertisement

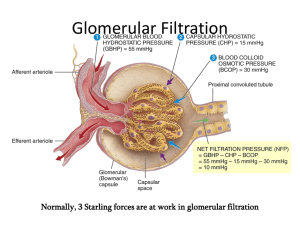
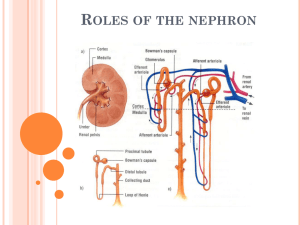
BIO2305 Renal Physiology Kidney Function Regulation of body fluid osmolality & volume: Excretion of water and Na+ is regulated in conjunction with cardiovascular, endocrine, & central nervous systems Regulation of electrolyte balance: Daily intake of inorganic ions Na+, K+, Cl-, HCO3-, H+, Ca2+, Mg+, & PO43Should be matched by daily excretion through kidneys Regulation of acid-base balance: Kidneys work in concert with lungs to regulate the pH in a narrow limits of buffers within body fluids Excretion of metabolic products & foreign substances: Urea from amino acid metabolism Uric acid from nucleic acids Creatinine from muscles Hemoglobin catabolism end products Hormone metabolites Foreign substances e.g., drugs, pesticides, & other chemicals ingested in the food Production and secretion of hormones: Renin – activates the Renin-Angiotensin-Aldosterone System (RAAS), thus regulating blood pressure & Na+, K+ balance Prostaglandins & Kinins – vasoactive, leading to modulation of renal blood flow & along with angiotensin II affect the systemic blood flow Example: Bradykinin is a vasodilator that lowers blood pressure and takes part in inflammation Erythropoietin (EPO) – stimulates erythropoiesis (red blood cell formation) by bone marrow Renal Anatomy Nephron – the functional unit of the kidney Renal Corpuscle: Bowman’s capsule Glomerulous (Glomerular capillaries) Promixal Convoluted Tubule (PCT) Loop of Henle (“hen/lee”) Distal Convoluted Tubule (DCT) Collecting duct 1 Functional Unit – Nephron Production of filtrate Reabsorption of organic nutrients Reabsorption of water and ions Secretion of waste products into tubular fluid Two Types of Nephrons Cortical nephrons ~85% of all nephrons Located in the cortex Juxtamedullary nephrons Closer to renal medulla Loops of Henle extend deep into renal pyramids 2 Cortical and Juxtamedullary Nephrons Blood Supply to the Kidneys Blood travels from afferent arteriole to capillaries within the Bowman’s capsule called the Glomerulus Blood leaves the renal corpuscle via the efferent arteriole Blood travels from efferent arteriole to peritubular capillaries and vasa recta, which eventually exit via the renal vein 3 Anatomical Review - Renal Corpuscle 4 Glomerular Filtration Glomerular filtrate is produced from blood plasma Filtration of molecules restricted on the basis of size and electrical charge Three Filtration Barriers that filtrate must pass through: Endothelial cell fenestrations of the glomerular capillary Basement membrane, the acellular gelatinous membrane made of collagen and glycoprotein (“lamina propria”) Filtration slits formed by interlocking podocyte foot processes Filtrate is similar to plasma in terms of concentrations of salts and of organic molecules (e.g., glucose, amino acids) except it is essentially protein-free Filtrate Composition Solute Characteristics: Neutral solutes Solutes without a charge Size: Radius smaller than 180 nm are freely filtered Radius between 180 and 360 nm are filtered to varying degrees Radius greater than 360 nm are not filtered Example: Serum albumin ~7 g/day filtered (out of ~70,000 g/day passing through glomeruli) a small plasma protein anionic 355 nm radius Glomerular Filtration Filtration is driven by Starling forces across the glomerular capillaries, and changes in these forces and in renal plasma flow alter the glomerular filtration rate (GFR) The glomerulus is more efficient than other capillary beds because: Its filtration membrane is significantly more permeable Glomerular blood pressure is higher resulting in a higher net filtration pressure Plasma proteins are not filtered and are used to maintain oncotic (colloid osmotic) pressure of the blood 5 Glomerular Filtration Rate (GFR) The Starling equation – equation that illustrates the role of hydrostatic and oncotic forces (Starling forces) in the movement of fluid across capillary membranes GFR - The total amount of filtrate formed per minute by the kidneys Filtration rate factors: Filtration Coefficient (Kf) - total surface area available for filtration and membrane permeability Net filtration pressure (NFP) – the resulting glomerular pressure that is responsible for filtrate formation GFR = Kf x NFP GFR is directly proportional to the NFP Changes in GFR normally result from changes in glomerular capillary blood pressure Forces Involved in Glomerular Filtration Net Filtration Pressure (NFP) - pressure responsible for filtrate formation NFP equals the glomerular hydrostatic pressure (HPg) minus the oncotic pressure of glomerular blood (OPg) plus capsular hydrostatic pressure (HPc) 6 Control of Kf (Filtration Coefficient) Filtration Coefficient (Kf) total filtration surface area and membrane permeability It is normally 12.5 mL/min/mm Hg (a constant) Kf is determined by 2 factors: The permeability of the capillary bed The surface area of the capillary bed Kf = permeability of glomerulus x filtration surface area Control of Kf (Filtration Coefficient) Mesangial cells Specialized smooth muscle cells wedged between glomerular capillaries Have contractile properties; influence capillary filtration by closing some of the capillaries Directly affect the filtration surface area Podocytes change size of filtration slits Kidney’s Receive 20-25% of CO Distribution of CO to Kidneys: ~ 22% of CO reaches the kidneys Filtration fraction: ~ 20% of the plasma that enters the glomerulus is filtered into the capsular space and enters the PCT (@ NFP of 10 mm HG) Male GFR: 125 mL/min Produce 180 L of filtrate/day Female GFR: 115 mL/min Produce 160 L of filtrate/day Required for adjustments and purification, not to supply kidney tissue 7 Calculate GFR Given: HR = 70 SV = 71 % of CO that goes to Kidneys = 22% % Hct = 45% Filtration Fraction = 20% Calculate GFR: Regulation of Glomerular Filtration If the GFR is too high, needed substances cannot be reabsorbed quickly enough and are lost in the urine If the GFR is too low - everything is reabsorbed, including lipid-soluble wastes that are normally disposed of GFR normally controlled by adjustment of glomerular BP Three mechanisms control the GFR: Renal autoregulation (intrinsic system) Neural controls (sympathetic nervous system) Hormonal mechanism (the renin-angiotensin system, ADH) Autoregulation of GFR (local) Under normal conditions (MAP = 80 –180 mm Hg) renal autoregulation maintains a nearly constant glomerular filtration rate Two mechanisms are in operation for autoregulation: Myogenic mechanism: Arterial pressure rises, afferent arteriole stretches Vascular smooth muscles contract Arteriole resistance offsets pressure increase; RBF (& hence GFR) remain constant. Tubuloglomerular feedback mechanism: Feedback loop consists of a flow rate (increased NaCl) sensing mechanism in macula densa of juxtaglomerular apparatus (JGA) Increased GFR (& RBF) triggers release of vasoactive signals Constricts afferent arteriole leading to a decreased GFR (& RBF) Decreased GFR (& RBF) triggers release of vasoactive signals Dilates afferent arteriole leading to a increaced GFR (& RBF) 8 Juxtaglomerular Apparatus Arteriole walls have juxtaglomerular (JG) cells - enlarged, smooth muscle cells, have secretory granules containing renin; act as mechanoreceptors Macula densa - tall, closely packed distal tubule cells, lie adjacent to JG cells; function as chemoreceptors or osmoreceptors Extrinsic Controls Neural Control: When the sympathetic nervous system is at rest: Renal blood vessels are maximally dilated Autoregulation mechanisms prevail Under stress: Norepinephrine is released by the sympathetic nervous system Epinephrine is released by the adrenal medulla Afferent arterioles constrict and filtration is inhibited The sympathetic nervous system also stimulates the renin-angiotensin mechanism Hormonal Control: A drop in filtration pressure stimulates the Juxtaglomerular apparatus (JGA) to release Renin (RAAS) and Erythropoietin Atria of the heart release Atrial Natriuretic Peptide (ANP) ADH from the neurohypophysis inhibit diuresis 9 Renin-Angiotensin-Aldosterone System (RAAS) Atrial Natriuretic Peptide (ANP) 10 Vasopressin (ADH) Mechanism 11 Other Factors Affecting Glomerular Filtration Prostaglandins (PGE2 and PGI2) Vasodilators produced in response to sympathetic stimulation and angiotensin II Are thought to prevent renal damage when peripheral resistance is increased Nitric oxide – vasodilator produced by the vascular endothelium Adenosine – vasoconstrictor of renal vasculature Endothelin – a powerful vasoconstrictor secreted by tubule cells Overview of Urine Formation 1) Glomerular Filtration Filtration mainly by Starling forces 2) Tubular Reabsorption of valuable substances from the tubular fluid back into blood Reabsorption by active or passive transport 3) Tubular Secretion of less desirable substances from the blood into the tubular fluid Secretion mainly by active transport Mass Balance: Amount Filtered – Amount Reabsorbed + Amount Secreted = Amount Excreted in Urine Reabsorption and Secretion Accomplished via diffusion, osmosis, active and facilitated transport Carrier proteins have a transport maximum (Tm) which determines renal threshold for reabsorption of substances in tubular fluid Transport Maximum (Tm): Reflects the number of carriers in the renal tubules available Exists for nearly every substance that is actively reabsorbed or secreted When the carriers are saturated, excess of that substance is excreted 12 Na+ Reabsorption Sodium reabsorption is almost always by active transport via a Na+-K+ pump Na+ reabsorption provides stored energy (Primary Active Transport) used in reabsorbing most other organic nutrients (Secondary Active Transport) Water reabsorption: Na+ is reabsorbed, and H2O follows (osmosis) Secondary Active Transport – Cotransport Na+-linked Secondary Active Transport (Cotransport System) Occurs mostly in PCT [Na+] gradient (stored energy) established by the Na+/K+ pump is used to move other solutes across membrane Reabsorption of: Glucose Ions Amino acids Healthy fasting blood glucose level (BGL): 70 – 110 mg/dL 13 Reabsorption: Transport Maximum (Tm) Non-reabsorbed Substances Substances are not reabsorbed if they: Lack carriers Are not lipid soluble Are too large to pass through membrane pores Creatinine and uric acid are the most important non-reabsorbed substances Urea is lipid soluble (nonpolar), moves freely across membranes, and is reabsorbed from the collecting duct to help increase osmolality in the countercurrent exchange mechanism Tubular Secretion Essentially reabsorption in reverse, where substances move from peritubular capillaries or tubule cells into filtrate Tubular secretion is important for: Disposing of substances not already in the filtrate Eliminating undesirable substances such as urea and uric acid Ridding the body of excess potassium ions Controlling blood pH 14 Reabsorption and Secretion at the PCT Glomerular filtration produces fluid similar to plasma without proteins The PCT Reabsorbs 60-70% of the filtrate produced: Sodium, all nutrients, cations, anions, (HCO3-), and water Lipid-soluble solutes Small proteins H+ secretion occurs in the PCT Transport Activities at the PCT Reabsorption and Secretion at the DCT DCT performs final adjustment of urine (last-minute tweaking of [solutes]) Active secretion or absorption Absorption of Na+ and ClSecretion of K+ and H+ based on blood pH Water is regulated by ADH (vasopressin) Na+, K+ regulated by aldosterone Na+ reabsorption K+ secretion Na+ and K+ generally move in opposite directions 15 Tubular Secretion and Solute Reabsorption at the DCT Control of Urine Volume & Concentration Urine volume and osmotic concentration are regulated by controlling water and sodium reabsorption Precise control allowed via facultative water reabsorption Osmolality The number of solute particles dissolved in 1 L of water Reflects the solution’s ability to cause osmosis Body fluids are measured in milliosmoles (mOsm) The kidneys keep the solute load of body fluids constant at about 300 mOsm This is accomplished by the Countercurrent Mechanism Countercurrent Mechanism Countercurrent Exchange System – the anatomical arrangement of vessels so that flow in one vessel is in the opposite direction from flow in the adjacent vessel Two Components: Filtrate in Loop of Henle that dip into the Renal Medulla Blood in Vasa Recta surrounding the Loop of Henle Solute concentrations in Loop of Henle and Vasa Recta range from 300 to 1200 mOsm Ions diffuse faster between fluids with a large difference in gas concentration (a high concentration gradient) 16 Countercurrent Multiplier Vasa Recta maintains medullary (ISF) osmotic gradient by receiving and carrying away H2O from the medulla Maintains the osmotic gradient Delivers blood to the cells in the area Loop of Henle: Descending Loop highly permeable to water; impermeable to solutes, Ascending Loop permeable to solutes; impermeable to water Collecting ducts in the deep medullary regions are permeable to urea, which helps maintain medullary hypertonicity Purpose of Countercurrent Exchange: To produce hyperosmotic (high [ion]) ISF in the medulla To produce a hyposmotic (dilute) filtrate in the absence of ADH To produce a hyperosmotic (concentrated) filtrate in the presence of ADH ADH (Vasopressin) ADH is used when the body needs to retain water. ADH inhibits diuresis and produces concentrated urine. Decreases the amount of water lost at the kidneys Elevates blood pressure Cells of the collecting duct require aquaporins for water permeability and osmosis ADH uses a G-protein/cAMP 2nd messenger system to stimulate the insertion of aquaporins into the collecting duct cell membrane. H2O then flow via osmosis toward the hyperosmotic medullary ISF and vasa recta 17 Presence of ADH In the presence of ADH, 99% of the water in filtrate is reabsorbed H2O moves via osmosis out of the tubule, toward the highly concentrated medullary ISF Urine osmolality can be as high as 1200 mOsm (4 x that of plasma) Absence of ADH In the absence of ADH, collecting ducts remain impermeable to water, and the filtrate within remains diluted Urine osmolality can be as low as 50 mOsm (1/6th that of plasma) 18 ADH and Aquaporins ADH uses a G-protein/cAMP 2nd messenger system to stimulate the insertion of aquaporins into the collecting duct cell membrane H2O then flows via osmosis toward the hypertonic medullary ISF and vasa recta Water Balance Reflex: Regulators of Vasopressin Release 19 Renal Clearance The volume of plasma that is cleared of a particular substance in a given time: RC = UV/P RC = renal clearance rate U = concentration (mg/ml) of the substance in urine V = flow rate of urine formation (ml/min) P = concentration of the same substance in plasma Renal clearance tests are used to: Determine the GFR Detect glomerular damage Follow the progress of diagnosed renal disease Creatinine Clearance Creatinine clearance is the amount of creatine in the urine, divided by the concentration in the blood plasma, over time. Glomerular filtration rate can be calculated by measuring any chemical that has a steady level in the blood, and is filtered but neither actively reabsorbed or secreted by the kidneys. Creatinine is used because it fulfills these requirements (though not perfectly), and it is produced naturally by the body. The result of this test is an important gauge used in assessing excretory function of the kidneys. Inulin and PAH Inulin is freely filtered at the glomerulus and is neither reabsorbed nor secreted. Therefore inulin clearance is a measure of GFR Substances which are filtered and reabsorbed will have lower clearances than inulin, Ux. Substances that are filtered and secreted will have greater clearances than inulin, Ux. Para-amino-hippuric acid (PAH) is freely filtered at the glomerulus and most of the remaining PAH is actively secreted into the tubule so that >90% of plasma is cleared of its PAH in one pass through the kidney. PAH can be used to measure the plasma flow through the kidneys = renal plasma flow. Excretion All Filtration Products that are not reabsorbed Excess ions, H2O, molecules, toxins, excess urea, "foreign molecules" Kidney Ureter Bladder Urethra out of body 20 Physical Characteristics of Urine Color and transparency Clear, pale to deep yellow (due to urochrome) Concentrated urine has a deeper yellow color Drugs, vitamin supplements, and diet can change the color of urine Cloudy urine may indicate infection of the urinary tract pH Slightly acidic (pH 6) with a range of 4.5 to 8.0 Diet can alter pH Specific gravity Ranges from 1.001 to 1.035 Is dependent on solute concentration Chemical Composition of Urine Urine is 95% water and 5% solutes Nitrogenous wastes include: Urea Uric acid Creatinine Other normal solutes include: Na+, K+, PO43-(phosphate), SO4(sulfate) Ca+2 (calcium), Mg+ (magnesium), HCO3- (bicarbonate) Abnormally high concentrations of any urinary constituents may indicate pathology Micturition From the kidneys urine flows down the ureters to the bladder propelled by peristaltic contraction of smooth muscle. The bladder is a balloon-like bag of smooth muscle (detrussor muscle), contraction of which empties bladder during micturition. Pressure-Volume curve of the bladder has a characteristic shape There is a long flat segment as the initial increments of urine enter the bladder and then a sudden sharp rise as the micturition reflex is triggered. Micturition (Voiding or Urination) Bladder can hold 250 - 400mL Greater volumes stretch bladder walls initiates micturition reflex: Spinal reflex: Parasympathetic stimulation causes bladder to contract Internal sphincter opens External sphincter relaxes due to inhibition 21 Urination: Micturition reflex Micturition (Voiding or Urination) 22