What is the acid
advertisement
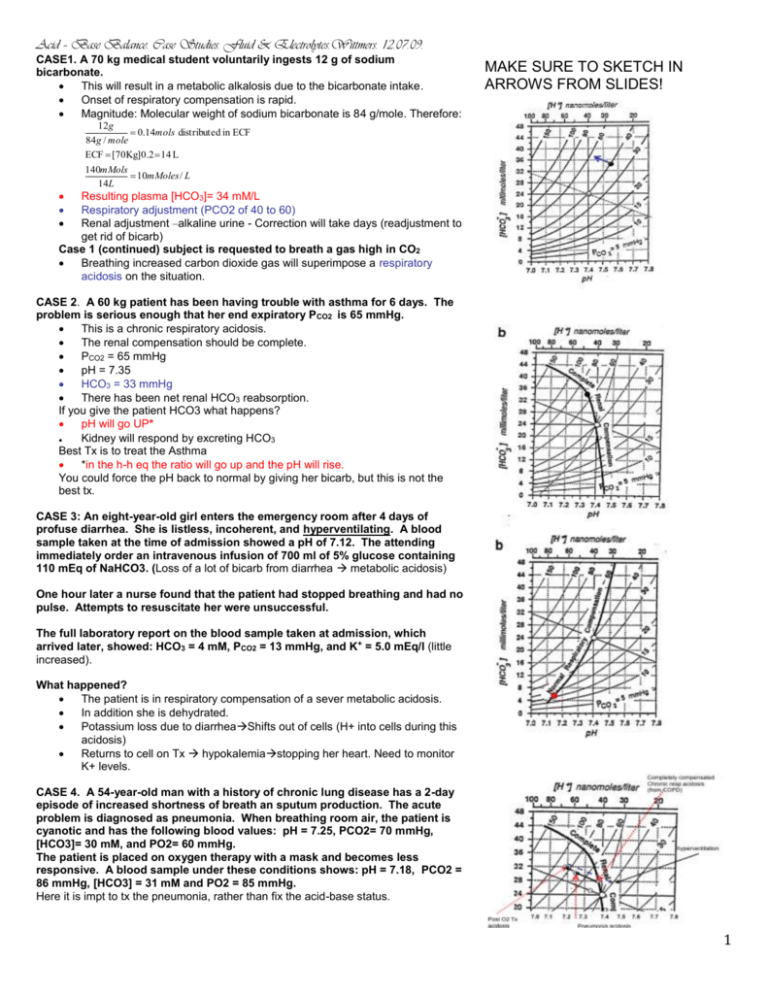
Acid – Base Balance. Case Studies. Fluid & Electrolytes.Wittmers. 12.07.09. CASE1. A 70 kg medical student voluntarily ingests 12 g of sodium bicarbonate. This will result in a metabolic alkalosis due to the bicarbonate intake. Onset of respiratory compensation is rapid. Magnitude: Molecular weight of sodium bicarbonate is 84 g/mole. Therefore: MAKE SURE TO SKETCH IN ARROWS FROM SLIDES! 12g 0.14mols distribute d in ECF 84g / mole ECF [70Kg]0.2 14 L 140mMols 10mMoles / L 14L Resulting plasma [HCO3]= 34 mM/L Respiratory adjustment (PCO2 of 40 to 60) Renal adjustment –alkaline urine - Correction will take days (readjustment to get rid of bicarb) Case 1 (continued) subject is requested to breath a gas high in CO2 Breathing increased carbon dioxide gas will superimpose a respiratory acidosis on the situation. CASE 2. A 60 kg patient has been having trouble with asthma for 6 days. The problem is serious enough that her end expiratory PCO2 is 65 mmHg. This is a chronic respiratory acidosis. The renal compensation should be complete. PCO2 = 65 mmHg pH = 7.35 HCO3 = 33 mmHg There has been net renal HCO3 reabsorption. If you give the patient HCO3 what happens? pH will go UP* Kidney will respond by excreting HCO3 Best Tx is to treat the Asthma *in the h-h eq the ratio will go up and the pH will rise. You could force the pH back to normal by giving her bicarb, but this is not the best tx. CASE 3: An eight-year-old girl enters the emergency room after 4 days of profuse diarrhea. She is listless, incoherent, and hyperventilating. A blood sample taken at the time of admission showed a pH of 7.12. The attending immediately order an intravenous infusion of 700 ml of 5% glucose containing 110 mEq of NaHCO3. (Loss of a lot of bicarb from diarrhea metabolic acidosis) One hour later a nurse found that the patient had stopped breathing and had no pulse. Attempts to resuscitate her were unsuccessful. The full laboratory report on the blood sample taken at admission, which arrived later, showed: HCO3 = 4 mM, PCO2 = 13 mmHg, and K+ = 5.0 mEq/l (little increased). What happened? The patient is in respiratory compensation of a sever metabolic acidosis. In addition she is dehydrated. Potassium loss due to diarrheaShifts out of cells (H+ into cells during this acidosis) Returns to cell on Tx hypokalemiastopping her heart. Need to monitor K+ levels. CASE 4. A 54-year-old man with a history of chronic lung disease has a 2-day episode of increased shortness of breath an sputum production. The acute problem is diagnosed as pneumonia. When breathing room air, the patient is cyanotic and has the following blood values: pH = 7.25, PCO2= 70 mmHg, [HCO3]= 30 mM, and PO2= 60 mmHg. The patient is placed on oxygen therapy with a mask and becomes less responsive. A blood sample under these conditions shows: pH = 7.18, PCO2 = 86 mmHg, [HCO3] = 31 mM and PO2 = 85 mmHg. Here it is impt to tx the pneumonia, rather than fix the acid-base status. 1 CASE 5. A 65-year old man has a history of smoking and hypertension., the later of which is being treated with a diuretic. He has the following arterial blood values: pH = 7.48, PCO2 = 51 mmHg, and HCO3= 36 mM. What is his acid-base status? Metabolic alkalosis With appropriate respiratory compensation. What is the most likely cause? The diuretic Tx could have lead to volume and K+ loss. Volume depletion leads to aldosterone secretion. This with hypokalemia stimulate cause kidney to inappropriately secret H+. CASE 6. A previously well patient enters the emergency room in a moribund state. Physical examination indicates severe pulmonary edema. Blood laboratory chemistries are: pH = 7.02, PCO2= 60 mmHg, HCO3 = 15 mM, and PO2= 40 mmHg. Is the acid-base status compatible with simple respiratory acidosis? If this was a pure respiratory problem the HCO3 would be high not low!! Would be along buffer line. This is a mixed disorder of rep & metabolic acidosis (can’t follow the arrows)!! Is the situation life threatening? Yes. CASE 7. The following laboratory results were obtained on one of your patients: Na+ = 140 mEq/l, K+= 4.2 mEq/l, and HCO3= 16 mEq/l. Would you immediately institute bicarbonate replacement therapy? Note: you don’t have a pH! No—the decreased bicarbonate could be due to a respiratory alkalosis. You would not give HCO3 to an alkalotic patient (hyperventilating down the curve). CASE 8. A 31-year-old man with a history of epilepsy has a grand mall seizure (Fixed acid from massive contraction of skeletal muscle (anaerobic)lactic acidosis). An arterial blood sample taken during the seizure shows: pH = 7.14, PCO2 = 45 mmHg, HCO3= 17 mM, and K+= 4.2 mEq/l. What is the acid-base disturbance? Mixed respiratory and metabolic acidosis. Does the patient need bicarbonate treatment? No need for HCO3 the lactic acid can be removed by the kidneys or metabolism. What will happen as the academia is corrected? Insufficient time for K+ movement. CASE 9. A 45-year-old patient with peptic ulcer disease reports 6 days of persistent vomiting. Her blood pressure is 100/60 mmHg and her jugular veins are flat. Initial laboratory data are: pH = 7.53, PCO2 = 53 mmHg, HCO3= 42 mM, K+ = 3.2 mEq/l, and urine pH = 5.0. What is the acid-base disturbance? Compensated metabolic alkalosis. Why is the urine acidic in this situation? Patient is hypovolemic and K+ depleted Urine problem is the same as in case 5(aldosterone –inappropriate H+ secretion.) Should this patient be treated? How? Soln: Fix the volume and the other things will correct in a few days. 2 CASE 10. A 45-year-old man with a long history of smoking reports more than a week of recurrent vomiting and has the following laboratory values: pH = 7.49, PCO2 = 55 mmHg, and HCO3 = 40 mM. What is the acid-base disturbance? Compensated metabolic alkalosis. What accounts for the elevation in PCO2? Due to the respiratory compensation alone. CASE 11. A 27-year-old man with insulin-dependent diabetes mellitus has not been taking his insulin and enters the hospital in a semi-comatose state. Admission arterial blood values are: pH = 7.15, PCO2= 20 mmHg, HCO3= 6 mM, and K+ = 4.5 mEq/l. Urine is positive for both glucose and ketones. What is the acid-base status? Metabolic acidosis with respiratory compensation. What treatment is necessary? Insulin –watch K+ (Inc K+ sec) Is K+ depletion likely here? K+ probably not needed –watch. CASE 12. A 50-year-old woman has chronic renal failure and has the following laboratory blood values: pH = 7.22, PCO2= 25 mmHg, HCO3= 10 mM, and K+ = 5.2 mEq/l. Metabolic acidosis w/ respiratory compensation. How does metabolic acidosis develop in renal failure? To get rid of normal acid production the fewer nephrons have to produce more acid per nephron. This needs an acid environment to be accomplished. What accounts for the elevation in plasma K+? Intra to extracellular K+ shift due to the acidosis. 3











