PURPOSE: 1.1 To lay down the procedure for calibration of HPLC
advertisement

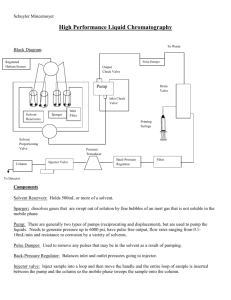
1.0 PURPOSE: 1.1 2.0 SCOPE. 2.1 3.0 Executive-QC ACCOUNTABILITY 4.1 5.0 This SOP is applicable for the Calibration of HPLC’S in QC / IPQC Laboratories. RESPONSIBILITY 3.1 4.0 To lay down the procedure for calibration of HPLC. Asst. Manager-QC PROCEDURE 5.1 Follow the respective SOP for operation and carry out the following tests for the calibration of a HPLC system as applicable. 5.1.1 FLOW RATE ACCURACY 5.1.2 GRADIENT COMPOSITION ACURACY 5.1.3 INJECTION VOLUME PRECISION 5.1.4 OVEN TEMPERATURE/SAMPLE CHILLER TEMPERATURE ACCURACY 5.1.5 WAVELENGTH ACCURACY 5.1.6 PRECISION /CARRY OVER 5.1.7 RESPONSE LINEARITY 5.2 FLOW RATE ACCURACY: 5.2.1 Flow rate accuracy shall be checked for 0.5 mL, 1.0mL and 2.0 mL/minute with the following chromatographic conditions. Column: Restriction capillary Mobile phase: HPLC grade water 5.2.1.1 Program the flow rate to 0.5 mL/minute and allow to stabilize. 5.2.1.2 Take a clean and dried measuring cylinder of 10mL capacity and weigh accurately(W1). 5.2.1.3 Take a calibrated stop watch. 5.2.1.4 Introduce the capillary outlet into the measuring cylinder and start the stop-watch simultaneously. 5.2.1.5 Stop the watch after collection of about 10 mL water in the cylinder and withdraw the capillary outlet simultaneously. Note the time elapsed as ‘t’. 5.2.1.6 Weigh the measuring cylinder containing water accurately (W2). Calculate the weight of water collected (W). 5.2.1.7 Calculate the volume (V) of water using the specific gravity of water at specified temperature as given below. Calculate the flow rate as follows; Flow rate (mL/minute)= V/t 5.2.1.8 Repeat steps 5.2.1.1 to 5.2.1.7 and determine the flow rate accuracy for 1.0 mL/min and 2.0 mL/min. 5.2.1.9 Record the details as per Format No. xxxxx TABLE 1 VOLUME OF 1 g OF WATER AT VARIOUS TEMPERATURES S.No TEMPERATURE( 0C) VOLUME (mL) 1 20 1.0027 2 22 1.0033 3 24 1.0037 4 26 1.0044 5 28 1.0047 6 30 1.0053 5.3 GRADIENT COMPOSITION ACCURACY 5.3.1 Determine the gradient composition accuracy using the following chromatographic conditions. 5.3.1.1 For Low pressure gradient system Pre-Purge Wavelength: 265 nm Flow: 2.0 mL/min Stop-time: 10.00 Solvent A: HPLC grade water. Solvent B:0. 5% Acetone in HPLC grade water. Timetable: Time 0.01 8.0 8.01 Gradient Run: Solvent B 100.0 100.0 0.0 Wavelength: 265 nm Solvent A: HPLC grade water. Solvent B:0. 5% Acetone in HPLC grade water. Flow: 2.0 mL/min Stop-time: 25 + 10 minute’s pre-purge. TABLE2 TIME TABLE TIME 1.00 1.01 5.00 5.01 8.00 8.01 11.00 11.01 14.00 14.01 17.00 17.01 20.00 20.01 25.00 SOLVENT B (%) 0.0 100.0 100.0 90.0 90.0 50.0 50.0 6.0 6.0 5.0 5.0 50.0 50.0 0.0 0.0 5.3.1.1 Prior to test gradient composition perform a pre-purge. 5.3.1.2 Perform the test in triplicate and Measure the average height of every step 5.3.1.3 Re-scale the height and noise from absorbance to composition (%B) values. 5.3.1.4 Calculate the composition accuracy for every step as the absolute difference between the rescaled measured step height and the corresponding gradient composition values. ACCEPTANCE CRITERIA : ± 1.500% 5.3.1.2 For High pressure gradient system Pre-Purge Wavelength: 254 nm Flow: 1.0 mL/min Stop-time: 10.00 Solvent A: HPLC grade water. Solvent B:0. 3% Acetone in HPLC grade water. Timetable: Time 0.01 10.0 Solvent B 10.0 10.0 Gradient Run: Wavelength: 254 nm Solvent A: HPLC grade water. Solvent B:0. 3% Acetone in HPLC grade water. Flow: 1.0 mL/min Stop-time: 50 min. TABLE3 TIME TABLE TIME 0.01 10.00 10.01 20.00 20.01 30.00 30.01 40.00 40.01 50.00 SOLVENT B (%) 10.0 10.0 50.0 50.0 90.0 90.0 100.0 100.0 0.0 0.0 5.3.1.1 Prior to test gradient composition perform a pre-purge. 5.3.1.2 Perform the test in triplicate and Measure the average height of every step 5.3.1.3 Re-scale the height and noise from absorbance to composition (%B) values. 5.3.1.4 Calculate the composition accuracy for every step as the absolute difference between the rescaled measured step height and the corresponding gradient composition values. ACCEPTANCE CRITERIA : ± 1.0% 5.4 INJECTION VOLUME ACCURACY 5.4.1 Injection volume precision shall be determined for 10L, 20 L, 50 L and 100L Injections. Chromatographic conditions: Mobile phase: HPLC grade water. Column: Restriction capillary. Run time: 1 min Flow: 1 mL/min 5.4.1.1 Fill four vials with HPLC grade water and cap them with septum. 5.4.1.2 Identify the vials and accurately weigh each of the vials separately. 5.4.1.3 Place the vials in the sample tray of the HPLC system and program an injection sequence as follows. VIAL Vial1 Vial2 Vial3 Vial4 TABLE3 INJECTION VOLUME 10L 20L 50L 100L REPETITIONS 5 5 5 5 5.4.1.4 After completion of the injection cycle weigh the vials accurately and determine the average injection volume for each nominal volume using the volume of water per gram at specified temperature as included in table 1. Record the details as per Format No. xxxxx ACCEPTANCE CRITERION: + 10.0% of programmed value. Note : INJECTION VOLUME ACCURACY is applicable only to HPLC system having autosampler / autoinjector. 5.5 OVEN TEMPERATURE/SAMPLE CHILLER TEMPERATURE ACCURACY 5.5.1 Oven temperature accuracy shall be checked at 300C, 400C and 500C. 5.5.2 Sample chiller accuracy shall be checked at the temperatures 100C, 150C and 200C. 5.5.3 Set the temperature of the oven /sample chiller to one of the above mentioned values, insert the temperature probe and allow to stabilize. After stabilization monitor the temperature at intervals of 5 minutes and note down three readings of the observed value. Repeat the procedure for the other nominal values and record the details as per Format No. xxxxx ACCEPTANCE CRITERION: + 20C of programmed value. 5.6 WAVELENGTH ACCURACY 5.6.1 Wavelength accuracy of UV detector Chromatographic conditions: Sample: 20 g/mL caffeine in water. Run time: 7 min. Wavelength program: TIME 0.0 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 7.0 WAVELENGTH 350 268 269 270 271 272 273 274 275 276 277 350 STOP 5.6.1.1 Fill the flow cell with caffeine standard solution by injecting directly into the detector inlet and start the program. 5.6.1.2 Record the peak response at various wavelengths as per Format Noxxxxx ACCEPTANCE CRITERION: Wavelength Maximum AT 273+ 1nm. NOTE : If the system has no wavelength programming option, than perform the test by selecting wavelength manually for each injection. 5.6.1 Wavelength accuracy of PDA detector 5.6.1.1 Fill the flow cell with caffeine standard solution by injecting Directly into the detector inlet and start the wavelength scan from 190 to 400nm.Record the details as per Format No. xxxxx ACCEPTANCE CRITERIA: Maxima at 205+ 1 nm and 273 + 1 nm. 5.7 INJECTION PRECISION / CARRY OVER 5.7.1 Perform injection precision and carry over as follows. Chromatographic conditions; Column: Restriction Capillary Flow: 1.00 mL/min Run time: 1.00 min Mobile Phase: HPLC grade water. Injection volume: 20.0L Wavelength:273 nm 5.7.1.1Inject 20.0L of mobile phase three times followed by six 20Linj. of Caffeine (20g/mL) in water and again inject one blank (mobile phase). 5.7.1.2 Calculate the injection precision for area as the % RSD of caffeine standard peak responses. 5.7.1.3 Calculate the injection carry over for area as peak response in the end blank injection with the peak response from the third blank injection subtracted as % of the response of the sixth Caffeine injection. Record the calibration details as per Format No. xxxxx ACCEPTANCE CRITERIA: 5.8 RESPONSE LINEARITY: Precision : RSD NMT 2.0% Carry over area: NMT 0.20%. 5.8.1 Determine the detector response linearity by injecting Caffeine standard solutions of concentrations 10g/mL, 20g/mL, 30g/mL, 50g/mL and 100g/mL in water. Chromatographic conditions: Same as 5.7.1 5.8.1.1 Inject Caffeine standard solutions of the above said concentrations. 5.8.1.2 Record the chromatogram and take the peak areas of responses as per Format No. xxxxx 5.8.1.3 Plot a linearity graph of concentration Vs peak response areas and Record the details as per Format No. xxxxx ACCEPTANCE CRITERION: Correlation Coefficient NLT 0.999 5.9 FREQUENCY: 5.9.1 Once in six months. END OF DOCUMENT