File
advertisement

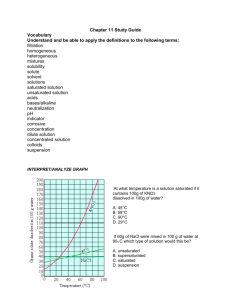
Final Examination Semester 1 Academic Year 2014 Course Code: SC21101 Subject: Science 1 Matthayomsuksa 1 Scores: 30 Marks Time: 60 minutes DIRECTIONS: 1. 2. 3. 4. This examination paper consists of 2 Parts; Multiple Choice and Critical Writing. 40 items of multiple choice and 2 writing essay questions 5 points each. Mark the correct answer on the answer sheet and briefly answer the critical writing questions. Hand in the examination paper and the answer sheet to the proctors. PART 1: MULTIPLE-CHOICE TEST 1. How the size of particles does affect the rate of solubility? 1. The bigger the size of the particle the faster the rate of solubility. 2. The smaller the size of the particle the slower the rate of solubility. 3. The bigger the size of the particle the slower the rate of solubility. 4. The smaller the size of the particle in higher temperature, the faster the rate of solubility. 2. What type of solution that contained little dissolved solute and able to dissolve a lot of solute in it? 1. Dilute Solution 2. Concentrated Solution 3. Saturated Solution 4. Mixed Solution 3. Which among the statement is NOT true for solution? 1. A solution is transparent and clear 2. A solution is homogeneous 3. The solute of a solution does not separate from the solvent when left to stand on its own 4. A solution is the end result of substances that turns into a compound 4. It is the systematic study of nature and how it affects us and our environment 1. Science 2.Technology 3.Scientific Method 4.Scientific Investigation 5. These are mixture containing substances that are insoluble in the solvent. 1. Colloids 2. Suspensions 3.Solutions 4. Mixtures 6. Which among the statements is NOT true for factors affecting Solubility? 1. Nature of pH 2. Nature of Solvent 3. Nature of Solute 4. Temperature 7. Which substance is MORE soluble in water? 1. Alcohol 2. Salt 3. Paint 4. Oil 8. Which one is NOT an organic solvent? 1. Acetone 2. Turpentine 3. Tap Water 4. Alcohol 9. How do you classify organic acids as organic in nature? 1. They are organic in nature because they are weak acids. 2. They are organic in nature because acids are naturally organic. 3. They are organic in nature because they come from minerals. 4. They are organic in nature because they basically have biological origin. Continue Page 2 Final Examination Science 1 (SC21101) Matthayomsuksa 1 2014 10. Which of the following is NOT true about the rate of dissolving? 1. The temperature affects the rate of dissolving 2. The rate of stirring affects the rate of dissolving 3. The time of the day affects the rate of dissolving 4. The size of the solute particles affects the rate of dissolving 11. It is a type of solution which is in excess of hydrogen ions. 1. Acid 2. Base 3. Alkali 4. Neutral 12. Orange Juice contains what type of acid? 1. Tartaric acid 2. Citric acid 3. Lactic acid 4. Malic acid 13. Grape contains what type of acid? 1. Tartaric acid 2. Citric acid 3. Lactic acid 4. Malic acid 14. Which among the answer is NOT true about acidic substance? 1. Taste sour 2. Corrosive 3. Turns the red litmus paper blue 4. Reacts with metal and gives 15. It is a solution that is soluble in water and has a pH above 7. 1. Acid 2. Alkali 3. Neutral 4. Both 2 and 3 are correct. 16. What is the pH level of egg? 1. pH 5 2. pH 6 3. pH 7 4. pH 8 17. Which of the following solution is base? 1. Ammonia 2. Hydrochloric Acid 3. Hot Coffee 4. Ice Tea 18. Which of the following solution is acidic? 1. Calcium Hydroxide 2. Soft Drinks 3. Ammonium Carbonate 4. Baking Soda 19. 20 g sugar is dissolved in a beaker of water. Choose the right equation below. 1. sugar + water = sugar solution 2. sugar + water → water solution 3. sugar + solution → sugar solution 4. sugar + water → sugar solution 20. Which of the following is NOT a property of Alkali? 1. Taste sour 2. It has soapy feel 3. Corrosive 4. Turns red litmus paper blue 21. Which one is true about detergents? 1. All detergents are base 2. All detergents are acid 3. Detergent can be alkali or acid 4. Detergent is soapy when it is base 22. It is a process that occurs when acid reacts with alkali to form salt and water. 1. Dilution 2. Concentration 3. Dissolution 4. Neutralization 23. Which one is a correct representation of the neutralization reaction with the following equation? 1. acid + salt → alkali + water 2. acid + water → alkali + salt 3. acid + alkali → salt + water 4. acid + water → salt + base 24. How do you prepare a 10 g/dm³ mean? 1. Prepare 10 grams of salt and 1 dm³ of distilled water. 2. Prepare 5 grams of salt and 5 dm³ of distilled water. 3. Prepare 9 grams of salt and 1 dm³ of distilled water. 4. Prepare 9 grams of salt and 1000 cm³ of distilled water. 25. What does 60 g/dm³ means? 1. It means 30 grams of solute and 30 dm³ of solvent. 2. It means 30 grams of solute and 30 cm³ of solvent. Continue Page 3 2 Final Examination Science 1 (SC21101) Matthayomsuksa 1 2014 3. It means 60 grams of solute and 1 dm³ of solvent. 4. It means 60 grams of solute and 1 cm³ of solvent. 26. Which one is NOT true about Neutralization? 1. Toothpaste neutralizes the acidic substances produce by the bacteria in the mouth 2. Shampoo neutralizes the skin that secretes acids 3. Conditioner neutralizes the residues of the shampoo 4. Coffee neutralizes the stomach that has hyperacidity 27. Which one is not an example of pH indicator? 1. Red Spinach 2. Phenolphthalein 3. pH scale 4. Universal indicator 28-31. Complete the following Table by selecting the right choice below: 1. Alcohol 2. Turpentine 3. Ethyl acetate & Acetone 4. Alcohol & Acetone Solute Solvent 28.Fragrant oil 29.Ink 30. Paint 31. Nail polish 32. The dissolved substance is called what? 1. Solution 2. Solvent 3. Solute 4. Dilute 33. A _____________ solution is a solution that can still dissolve more solute. 1. Dilute 2. Concentrated 3. Saturated 4. Both 1 and 3 34. A _____________ solution is a solution that can only dissolve a little bit solute. 1. Dilute 2. Concentrated 3. Saturated 4. Both 1 and 3 35. A ______________ solution is a solution that cannot dissolve any more solute. 1. Dilute 2. Concentrated 3. Saturated 4. Both 1 and 3 36. How is the effect of acids on litmus paper? 1. Blue litmus paper turns red. 2. Red litmus paper turns blue. 3. Blue litmus paper remains blue. 4. None of the above is correct. 37. Which of the following is NOT an organic acid? 1. Formic acid 2. Sulfuric Acid 3. Acetic Acid 4. Citric Acid 38. Which among the substance has the highest pH level? 1. Detergent 2. Oven Cleaner 3. Baking Soda 4. Toothpaste 39. Which of the following explanation that correctly determined the pH of the substance by using phenolphthalein? 1. Phenolphthalein turns white when the pH of a substance is in the range of 1-9. 2. Phenolphthalein turns pink when the pH of a substance is in the range of 1-9. 3. Phenolphthalein turns colorless when the pH of a substance is in the range of 10-14. 4. Phenolphthalein turns pink when the pH of a substance is in the range of 10-14. 40. Why is it incorrect to use cold water to dissolve the sugar cube but water at room temperature to dissolved powdered sugar when comparing the rate of dissolving sugar in water? 1. Because cold water has a high kinetic energy and it would affect the comparison test. 2. Because cold water freezes the powdered sugar to become a cube too. 3. Because temperature is a variable that affects the comparison test. 4. All of the above. 3 Continue Page 4 Final Examination Science 1 (SC21101) NAME:________________________________________ Matthayomsuksa 1 CLASS:________ 2014 NUMBER:_______ PART 2: CRITICAL WRITING (5 Points each) 1. Ms. Nalin wanted to cook some carrots because her father was hungry. She put 4 whole carrots in a pot of water under a small flame. It took half an hour for the carrots to turn soft. Give two suggestions to reduce the cooking time. 2. Explain how does neutralization helps in curing stomachache. 4 Final Examination Science 1 (SC21101) Matthayomsuksa 1 2014 ANSWER KEY 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 3 1 4 1 2 1 2 3 4 3 1 2 1 3 2 4 1 2 4 1 3 4 3 1 1 4 1 1 4 2 3 3 1 2 3 1 2 2 4 3 PART 2: 1. Ms. Nalin should increase the temperature or the flame, and she should cut the carrots into smaller pieces. 2. Weak alkali such as milk of magnesia can be used to relieve stomach pain which is caused by over-secretion of acid. Or take any safe antacid tablet/fluid to neutralize the acidity that causes stomachache. 5 Final Examination Science 1 (SC21101) 6 Matthayomsuksa 1 2014