Electronic Supplementary Material
advertisement
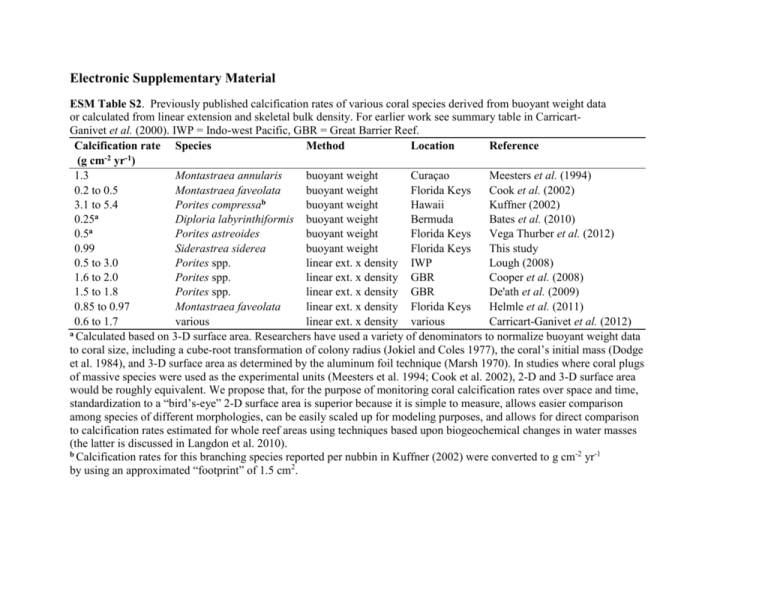
Electronic Supplementary Material ESM Table S2. Previously published calcification rates of various coral species derived from buoyant weight data or calculated from linear extension and skeletal bulk density. For earlier work see summary table in CarricartGanivet et al. (2000). IWP = Indo-west Pacific, GBR = Great Barrier Reef. Calcification rate Species Method Location Reference -2 -1 (g cm yr ) 1.3 Montastraea annularis buoyant weight Curaçao Meesters et al. (1994) 0.2 to 0.5 Montastraea faveolata buoyant weight Florida Keys Cook et al. (2002) 3.1 to 5.4 Porites compressab buoyant weight Hawaii Kuffner (2002) a 0.25 Diploria labyrinthiformis buoyant weight Bermuda Bates et al. (2010) a 0.5 Porites astreoides buoyant weight Florida Keys Vega Thurber et al. (2012) 0.99 Siderastrea siderea buoyant weight Florida Keys This study 0.5 to 3.0 Porites spp. linear ext. x density IWP Lough (2008) 1.6 to 2.0 Porites spp. linear ext. x density GBR Cooper et al. (2008) 1.5 to 1.8 Porites spp. linear ext. x density GBR De'ath et al. (2009) 0.85 to 0.97 Montastraea faveolata linear ext. x density Florida Keys Helmle et al. (2011) 0.6 to 1.7 various linear ext. x density various Carricart-Ganivet et al. (2012) a Calculated based on 3-D surface area. Researchers have used a variety of denominators to normalize buoyant weight data to coral size, including a cube-root transformation of colony radius (Jokiel and Coles 1977), the coral’s initial mass (Dodge et al. 1984), and 3-D surface area as determined by the aluminum foil technique (Marsh 1970). In studies where coral plugs of massive species were used as the experimental units (Meesters et al. 1994; Cook et al. 2002), 2-D and 3-D surface area would be roughly equivalent. We propose that, for the purpose of monitoring coral calcification rates over space and time, standardization to a “bird’s-eye” 2-D surface area is superior because it is simple to measure, allows easier comparison among species of different morphologies, can be easily scaled up for modeling purposes, and allows for direct comparison to calcification rates estimated for whole reef areas using techniques based upon biogeochemical changes in water masses (the latter is discussed in Langdon et al. 2010). b Calcification rates for this branching species reported per nubbin in Kuffner (2002) were converted to g cm-2 yr-1 by using an approximated “footprint” of 1.5 cm2. References Cited in ESM Table S2 Bates NR, Amat A, Andersson AJ (2010) Feedbacks and responses of coral calcification on the Bermuda reef system to seasonal changes in biological processes and ocean acidification. Biogeosciences 7: 2509-2530 Carricart-Ganivet JP, Beltran-Torres AU, Merino M, Ruiz-Zarate MA (2000) Skeletal extension, density and calcification rate of the reef building coral Montastraea annularis (Ellis and Solander) in the Mexican Caribbean. Bull Mar Sci 66: 215-224 Carricart-Ganivet JP, Cabanillas-Teran N, Cruz-Ortega I, Blanchon P (2012) Sensitivity of calcification to thermal stress varies among genera of massive reef-building corals. PLoS One 7: e32859. Cook CB, Mueller EM, Ferrier MD, Annis E (2002) The influence of nearshore waters on corals of the Florida reef tract. In: Porter JW, Porter KG (eds) The Everglades, Florida Bay, and coral reefs of the Florida Keys: An ecosystem sourcebook. CRC Press, Boca Raton, FL, pp 771-788 Cooper TF, De'ath G, Fabricius KE, Lough JM (2008) Declining coral calcification in massive Porites in two nearshore regions of the northern Great Barrier Reef. Global Change Biol 14: 529-538 De'ath G, Lough JM, Fabricius KE (2009) Declining coral calcification on the Great Barrier Reef. Science 323: 116-119 Dodge RE, Wyers SC, Frith HR, Knap AH, Smith SR, Cook CB, Sleeter TD (1984) Coral calcification rates by the buoyant weight technique: effects of alizarin staining. J Exp Mar Biol Ecol 75: 217-232 Helmle KP, Dodge RE, Swart PK, Gledhill DK, Eakin CM (2011) Growth rates of Florida corals from 1937 to 1996 and their response to climate change. Nature Comm 2: 215 Jokiel PL, Coles SL (1977) Effects of temperature on the mortality and growth of Hawaiian reef corals. Mar Biol 43: 201-208 Kuffner IB (2002) Effects of ultraviolet radiation and water motion on the reef coral, Porites compressa Dana: a transplantation experiment. J Exp Mar Biol Ecol 270: 147-169 Langdon C, Gattuso JP, Andersson AJ (2010) Measurements of calcification and dissolution of benthic organisms and communities. In: Riebesell U, Fabry VJ, Hansson L, Gattuso JP (eds) Guide to best practices for ocean acidification research and data reporting. Publications Office of the European Union, Luxembourg, pp 213-230 Lough JM (2008) Coral calcification from skeletal records revisited. Mar Ecol Prog Ser 373: 257-264 Marsh JA, Jr (1970) Primary productivity of reef-building calcareous red algae. Ecology 51: 255-263 Meesters EH, Noordeloos M, Bak RPM (1994) Damage and regeneration: links to growth in the reef-building coral Montastrea annularis. Mar Ecol Prog Ser 112: 119-128 Vega Thurber R, Burkepile DE, Correa MS, Thurber AR, Shantz AA, Welsh R, Pritchard C, Rosales S(2012) Macroalgae decrease growth and alter microbial community structure of the reef-building coral, Porites astreoides. PLoS One 7: e44246