Notes
advertisement

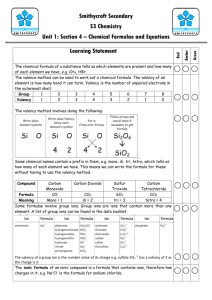
NAT 5 Chemistry Calculations Chemical Formula Working out the correct chemical formulae is important because it is the first stage in some calculation Chemical Formulae from Prefix Some help in writing the correct chemical formulae is often given by the following prefixes: mono di tri tetra - one - two - three - four Examples: Carbon monoxide CO Sulphur dioxide Phosphorus trichloride Dinitrogen tetraoxide SO2 PCl3 N2O4 Write chemical formulae for the following five compounds: 1. Carbon dioxide 2. Nitrogen dioxide 3. Carbon disulphide 4. Carbon tetrachloride 5. Nitrogen monoxide The cross-over method The valency of an element tells us how many atoms of another element in a compound that particular atom will combine with. Remember, hydrogen has a valency of one Transition Metal Elements Transition metal elements can have more than one valency. The valency to be used for any transition metal element is written in Roman Numerals, in brackets, after the name of the transition metal element. Roman Numerals: (i) = (ii) = (iii) = (iv) = 1 2 3 4 Valency of transition metal elements The cross-over method for –ide compounds Example 1: Calcium chloride Elements Valency calcium 2 chlorine 8-7 = 1 Write the symbol with the valency number at the top right hand corner of the symbol. Cross-over Reduce ratio Chemical formula Ca2 1 1 Cl1 2 2 CaCl2 Example 2: Aluminium oxide Symbols/valency Cross-over Reduce ratio Al3 2 2 Chemical formula O2 3 3 Al2O3 Write chemical formulae for the following ten –ide compounds: 6. 7. 8. 9. 10. Sodium chloride Magnesium sulphide Aluminium fluoride Calcium nitride Zinc(ii) bromide 11. 12. 13. 14. 15. Nickel(ii) chloride Potassium sulphide Lithium nitride Iron(iii) oxide Magnesium nitride The cross-over method for –ite and -ate compounds Example 1: Copper(ii) Sulphate The sulphate ion is a complex ion. Check the correct formula and ion charge of any complex ion in the table, ‘formulae of selected ions…’ on page 4 of the SQA data booklet. SO42- Sulphate ion is Place the formula of the ion in brackets (SO4) with the valency of the ion represented by the charge on the ion i.e. 2. Elements/complex ions copper(ii) Valency Sulphate ion 2 2 Write the symbol with the valency number at the top right hand corner of the symbol. Symbol/valency Cross-over Reduce ratio Chemical formula Example 2: Symbols/valency Cross-over Reduce ratio Chemical formula Cu2 2 1 (SO4)2 2 1 Cu(SO4) or CuSO4 Ammonium Phosphate (NH4)1 3 3 (PO4)3 1 1 (NH4)3 PO4 Write chemical formulae for the following ten –ite/ate compounds: 16. 17. 18. 19. 20. Ammonium nitrate Magnesium phosphate Iron(iii) sulphate Calcium nitrate Boron carbonate 21. 22. 23. 24. 25. Calcium hydroxide Potassium sulphate Lithium carbonate Calcium sulphite Magnesium nitrate Ionic Formulae Ionic Formulae are the chemical formulae of ionic compound including the charges on the ions in the compound. Example 1: Calcium chloride Elements calcium Ion Ca2+ Valency 2 chlorine Cl8-7 = 1 Write the symbol of the ion with the valency number at the top right hand corner of the brackets surrounding the ion. (Ca2+)2 Cross-over Reduce ratio (Cl-)1 1 1 2 2 Ca2+(Cl-)2 Chemical formula Example 2: Ion/valency Ammonium Phosphate (NH4+)1 (PO43-)3 -over 3 1 Reduce ratio 3 1 Chemical formula (NH4+)3 PO43- Write ionic formulae for the following ten –ite/ate compounds: 26. 27. 28. 29. 30. Ammonium nitrate Magnesium phosphate Iron(iii) sulphate Calcium nitrate Boron carbonate 31. 32. 33. 34. 35. Calcium hydroxide Potassium sulphate Lithium carbonate Calcium sulphite Magnesium nitrate Chemical Equations Balanced equations indicate the mass of reactants and products involved in a particular reaction. Word Equations. Chemical reactions can be represented by chemical equations. These equations inform scientists of what is happening during a chemical reaction. Example: Sodium reacts with oxygen to produce sodium oxide. sodium + oxygen → sodium oxide Write word equations for the following reactions: 36 When magnesium (a grey metal) burns in air it gives out a bright light and lots of heat. A white powder called magnesium oxide is also produced. 37 When the colourless gas hydrogen is and the green gas chlorine are mixed and a bright light is shone on the mixture, an explosion occurs and white fumes of hydrogen chloride are formed. 38 When chalk (calcium carbonate) is added to sulphuric acid, calcium sulphate and water are produced. Carbon dioxide is also produced. 39 When grey magnesium metal is added to a blue solution of copper(ii) sulphate, brown copper solid forms on the magnesium and the blue solution fades to a clear solution of magnesium sulphate. 40 When hydrochloric acid is added to sodium hydroxide no change was seen to have taken place. The products of this reaction are sodium chloride and water. Formula Equations. Formula equations are a short hand way of showing chemicals reacting and being produced using their chemical formulae. Example: Copper reacts with silver(i) nitrate to form silver and copper(ii) nitrate. Word Equation Copper + silver(i) nitrate → silver + copper(ii) nitrate Formula equation Cu + AgNO3 → Ag + Cu(NO3)2 Write formula equations for each of the following reactions: 41 magnesium + sulphur dioxide → magnesium oxide + sulphur 42 calcium + water → calcium hydroxide + hydrogen 43 calcium hydroxide + sulphuric acid → calcium sulphate + water 44 potassium carbonate + hydrochloric acid → potassium chloride + water + carbon dioxide 45 potassium sulphate solution + barium nitrate solution → potassium nitrate solution + barium sulphate solution Balanced Equations. A formula equation is balanced if the number and type of atoms on the left hand side of the equation exactly equal the number and type of atoms on the right hand side of the equation. Example: When hydrogen reacts with chlorine, hydrogen chloride is formed. Word equation hydrogen + chlorine → hydrogen chloride Formula equation H2 + Cl2 → HCl Balanced equation H2 + Cl2 → 2HCl Balance the following chemical equations: 46. Mg(s) + O2(g) → MgO(s) 47. NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + H2O(l) 48. AgNO3(aq) + BaCl2(aq) → Ba(NO3)2(aq) + AgCl(s) 49. Na(s) + H2O(l) → NaOH(aq) + H2(g) 50. Al(s) + Cl2(g) → AlCl3(s)