pmic12190-sup-0001
advertisement

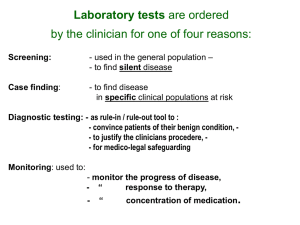
Supplemental Methods and Results. MALDI-TOF-MS Profiling Comparison, Optimization, and Reproducibility. Methods: Our goal for this study was to determine the optimal serum volume for use in our 2D-MALDI-MS approach, and to determine the level of reproducibility using the optimized volume, while also carrying out a direct comparison to previously published MALDI-MS profiling reports. To do this, we first took a single human volunteer serum sample and carried out our 2D workflow using 25µl, 50µl, 100µl, and 200µl of serum in triplicate (n=3). We then applied the optimized volume of serum at 100µl and ran the same sample through eight experimental replicates (n=8) to compare the reproducibility and efficiency of each fraction. Finally, we compared three different previously published analyses to our 2D approach (n=3): 1) dilute and shoot (DS – 1µl serum), 2) C18 cleanup (10µl serum), and 3) 1D SCX/ C18 cleanup (50µl serum) [1-3]. Briefly, the DS approach only required that the ~1µl of serum be diluted in the matrix and analyzed directly, the C18 cleanup approach required ~10µl of serum, utilizing a solid core C18 resin that absorbs the proteins/ peptides allowing desalting, while enriching LMW proteins over HMW proteins. For the 1D/ cleanup fractionation approach we used 50µl of serum fractionated as indicated for the 2D approach, followed by desalting using the same reverse phase resin with a single 80% organic bump prior to analysis by MALDIMS. All spectra were converted to .txt files, analyzed, and the features were extracted as indicated in the main methods section. For analysis of the reproducibility experiments, normalized feature intensities were converted to natural log (ln) values (yaxis) and plotted against the m/z values (x-axis) using Origin v.7.5 (OriginLab, Northampton, MA), and Pearson’s r coefficients (R2) were also calculated between ion intensities for each individual analysis versus the average. Results: We first compared increasing volumes of a single human serum sample (25µl, 50µl, 100µl, 200µl) using our 2D MALDI-MS approach (average values; n=3), followed by carrying out an experimental replicate study (n=8) on the optimized volume of 100µl, and then compared that optimized volume to other more common and previously published MALDI-MS profiling methods (average values; n=3) (Supplimental Figure 1a). When comparing increasing volumes of sera with the 2D MALDI-MS approach, 25µl of serum generated 351 features, 50µl generated 566 features, 100µl generated 637 features, and 200µl generated 671 features. Based on this data we felt that 100µl of serum was optimal when considering the lack of serum often provided by a single animal combined with the few additional features generated by 200µl compared to 100µl of serum. The optimal volume was then further used to measure experimental robustness. To accomplish this task, we generated ln-ln plots to measure any deviation in linearity, while measuring the number of features along with %CV for each fraction grouped by % organic in the second dimension. The detailed summation of this data is indicated (Supplemental Figure 1b, c, d), with an average of ~50 features generated per fraction, an average CV of ~0.2, and average R2 of ~0.99. When comparing across a few of the more common MALDI-MS profiling methods, the DS approach was by far the most straightforward, while requiring the least amount of serum at 1µl, presenting with an average of 37 features. The C18 cleanup approach was also very straightforward, required only 10µl of serum, and presented with an average of 63 features. The 1D/ cleanup approach was considerably more involved, and required 25-50µl of serum, generating an average of 257 features. The optimized 2D MALDI-MS approach required the most serum at 100µl, and was also the most involved, generating a significant increase to 637 features on average. While the absolute number of features may differ from previously published data, we applied very strict methods for data analysis and feature extraction, applied equally across all of these experiments so that an accurate comparison could be applied. Protein Identification by a Top-Down Directed Workflow. Sera from the same set of animals studied above were sub-pooled (6-7 animals per sub-pool, one sub-pool per genotype), and 100μL of each sub-pooled serum specimen were processed via a top-down-directed (TDD) approach used to carry out feature identification (Supplemental Figure 2) [4-6]. Each sample was depleted of the seven serologically most abundant proteins using ProteomeLabTM IgY-R7 LC10 Proteome Partitioning kit (Cat.# A25403-AA, Beckman Coulter) following manufacturers’ instructions and in-line with a LC-10AT HPLC (Shimadzu, Columbia, MD). The FT and bound fractions were collected separately by hand into 50mL conical tubes. The FT fractions were then quantified using a BCA Protein Assay Reagent (Cat.#, 23225, Pierce, Thermo Scientific, Rockford, IL) as per manufacturers’ instructions, and 200ug of protein were concentrated and desalted using a Protein C4 Macrotrap (Cat.# TR1/25108/53, Michrom Bioresources Inc., Auburn, California) as per manufacturers’ instructions, followed by dilution to 20% ACN in 50mM NaOAc, pH3.8 and fractionated using a SCX Macrotrap (Cat.# TR1/25108/55, Michrom Bioresources Inc., Auburn, California) with the same salt bumps as described for the 2D MALDI-MS approach. Each fraction for each genotype were then loaded onto a BioBasic4 column 150mm x 2.5mm (Cat#.72305-153030, Thermo Fisher, Waltham, MA) and further separated into 96 fractions using a LC-10AT HPLC (Shimadzu, Columia, MD) at a flow rate of 0.4mL/min and collected at 0.5min/fraction in-line with an automated fraction collector (FC203B, Gilson, Middletown, WI), using Whatman™ 96-Well Polypropylene UniPlate™ Microplate, v-shaped, natural, 250µl (Cat.# 7701-5250, Thermo Fisher, Waltham, MA). Mobile phases used included solvent A (0.1%TFA in ddH2O) and solvent B (0.1%TFA in ACN). The instrument settings were as follows: 0-2min 8% solvent B, 2-6min 18% solvent B, 6-40min 42% solvent B, 40-45min 65% solvent B, 45-48min 90% solvent B, 50-55min 0% solvent B. All 96 fractions were taken over the first 48 minutes, and concentrated to near dryness using a Savant SpeedVac (Thermo Fisher, Waltham, MA) and diluted to 20μL/ fraction with 0.1% TFA in 50:50 ACN:ddH2O out of which 1.5μL per fraction was spotted on a MALDI target plate as described for the 2D MALDI-MS approach, with external standards spotted in the middle of every eight spots, and analyzed with the UltraflexIII (Bruker Daltonics, Billerica, MA, USA) using WARP-LC (Bruker Daltonics, Billerica, MA, USA). Each fraction containing features of interest were identified using SurveyViewer (Bruker Daltonics, Billerica, MA, USA), and were loaded onto Novex® 10-20% 1.0mm Tricine gels (Cat.# EC6625BOX, Life Technologies, Carlesbad, CA). The gels were stained with SYPRO Ruby (Cat#S-12000, Life Technologies, Carlesbad, CA) as per manufacturers’ instructions. Bands corresponding to the proteins of interest were excised and digested with Trypsin Gold Mass Spectrometry Grade (Cat.# V5280, Pierce, Thermo Scientific, Rockford, IL) as per manufacturers’ instructions. Digests were then reconstituted in 10μL of 0.1%FA in 5:95 ACN:ddH2O, and analyzed using an LTQ XL linear ion trap mass spectrometer fitted with a nano ESI source (Thermo Fisher, San Jose, CA). The LCMS settings were described previously [5]. The data was searched using Mascot (v2.1, .mgf files, Matrix Science, Boston, MA), which was set for two missed cleavages, a precursor mass window of 0.45 Da, Tryptic, static modification C at 57.0293, and a variable modification M at 15.9949. For the fragment-ion mass tolerance, the Mascot default of 0.8Da was used. The searches were performed with a mouse subset of the UniRef100 database, which included common contaminants such as digestion enzymes and human keratins. All proteins identified using this approach are discussed in the results section and listed with their corresponding m/z values in Supplemental Table 2. 1. 2. 3. 4. 5. 6. Taguchi, F., et al., Mass spectrometry to classify non-small-cell lung cancer patients for clinical outcome after treatment with epidermal growth factor receptor tyrosine kinase inhibitors: a multicohort cross-institutional study. J Natl Cancer Inst, 2007. 99(11): p. 838-46. Villanueva, J., et al., Serum peptide profiling by magnetic particle-assisted, automated sample processing and MALDI-TOF mass spectrometry. Anal Chem, 2004. 76(6): p. 1560-70. Mobley, J.A., et al., Monitoring the serological proteome: the latest modality in prostate cancer detection. J Urol, 2004. 172(1): p. 331-7. Chaurand, P., et al., New developments in profiling and imaging of proteins from tissue sections by MALDI mass spectrometry. J Proteome Res, 2006. 5(11): p. 2889-900. Kojima, K., et al., Applying proteomic-based biomarker tools for the accurate diagnosis of pancreatic cancer. J Gastrointest Surg, 2008. 12(10): p. 1683-90. Lam, Y.W., et al., Mass profiling-directed isolation and identification of a stagespecific serologic protein biomarker of advanced prostate cancer. Proteomics, 2005. 5(11): p. 2927-38. Supplemental Figure 1. 2D-MALDI-MS Optimization & Reproducibility (a; n=3, bd; n=8). a) the number of observed features with S/N>3 are indicated vs. method applied (top) including 1) “DS” dilute and shoot, where serum is combined directly with MALDI matrix, 2) “Clean up”, where serum is diluted 1:10 with 0.1%TFA prior to solid phase extraction (SPE) with a C18 resin, 3) “1D”, where serum is partitioned using four salt bumps with a strong cation exchange (SCX) resin, followed by a SPE desalting step with a C18 resin, and 4) “2D” as outlined in Figure 2, where the volume used was also optimized at 100µl (bottom). b-d))The number of features observed and %CV’s for each of 12 SPE fractions is indicated for the 2D MALDI-TOF-MS approach using 100µL of serum, and the Pierson’s coefficient measurement for the combined SCX fractions following reverse fractionation is also indicated for the three organic extractions at 35%, 45%, and 80% respectively. Supplemental Figure 2. Top-Down-Directed (TDD) Workflow for Protein ID. a) LCMALDI-MS directed “intact” protein identification workflow, b) visual inspection of each statistically significant feature is important prior to purification and characterization. An example at 6,568, m/z is shown, c) following top-down-directed LC-MALDI-MS, and 1D PAGE, the molecular weight (MW) region of interest was digested with trypsin for nLCMS analysis and protein identification. Supplemental Figure 3. Network Analysis. One of the top networks identified using systems biology analysis was related to the acute-phase and innate immune response. This analysis is based on previously published associations with the biomarkers directly identified in this study. There is a strong indication that the JAK/STAT pathway is activated, and while in this figure it is indicated that IL-6 is the highlighted driving force, this pathway could be activated similarly through alternative mechanisms. Supp. Table 1. Summary of Pancreatic Histopathology. Histologic evaluation was carried out on FFPE H&E sections taken from the pancreas of each mouse used in this study. Genotype ID Pdx-Cre-1 Pdx-Cre-2 Pdx-Cre-3 Pdx-Cre-4 Pdx-Cre-5 Pdx-Cre-6 Pdx-Cre-7 Pdx-Cre-8 Pdx-Cre/ Ras-1 Pdx-Cre/ Ras-2 Pdx-Cre/ Ras-3 Pdx-Cre/ Ras-4 Pdx-Cre/ Ras-5 Pdx-Cre/ Ras-6 Pdx-Cre/ Ras-7 Pdx-Cre/ Ras-8 Ducts Observed 7 9 7 Abnormal Ducts 0 0 0 6 7 8 9 9 7 17 21 12 10 11 12 6 0 0 0 0 0 0 5 7 1 0 1 5 1 Pathology Non-observed Non-observed Hypertrophy of Islet Cells Non-observed Non-observed Non-observed Non-observed Non-observed Non-observed PanIN(2) A-typical/ Unclear PanIN(1) Non-observed A-typical/ Unclear PanIN(1) PanIN(2)