Water Reactive Chemicals SOP
advertisement

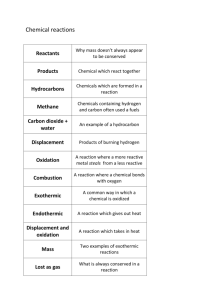
Standard Operating Procedure Settlement Class: Water Reactive Chemicals Common groups: Grignard reagents (RMgX); Alkali metals (Li, Na, K); Alkali metal amides; Alkali metal hydrides (Lithium aluminum hydride); Metal alkyls (Lithium and aluminum alkyls); Chlorosilanes; Halides of nonmetals (BCl3, BF3, PCl3, PCl5, SiCl4, S2CL2); Inorganic acid halides (POCl3, SOCl2, SO2Cl2); Anhydrous metal halides (AlCl3, AlBrx, TiCl4, ZrCl4, SnCl4); Organic acid halides; and anhydrides of low molecular weight. See Appendix A for the actual settlement list pertaining to this hazard family/class. NOTE: Water reactive material may also present additional hazards such as corrosivity or toxicity. These materials may also have pyrophoric properties. Print a copy and insert into your Laboratory Safety Manual and Chemical Hygiene Plan. Department: Chemistry Date SOP was written: Date SOP was approved by PI/lab supervisor: Principal Investigator: Internal Lab Safety Coordinator/Lab Manager: Lab Phone: 12/5/2012 Office Phone: 510-643-6312 Emergency Contact: Richmond Sarpong, 626-644-2407 (Name and Phone Number) Latimer Hall: 834, 836, 837, 838, 839, 842, 847, 849, 907 (Building/Room Number) Location(s) covered by this SOP: Type of SOP: Process 1/13/2013 Richmond Sarpong Rebecca Murphy 510-643-2485 Hazardous Chemical Hazardous Class Purpose This is a chemical class SOP. Some uses of the chemicals in this class require specific SOPs. Chemical-specific SDSs and all relevant SOPs must be reviewed prior to use of any chemical in this class. A complete list of the water reactive chemicals from the Settlement Agreement can be found in Appendix A. Water Reactive Chemicals 1 1/2/2013 Physical & Chemical Properties/Definition of Chemical Group CAS#: List here Class: List here NOTE: this may vary from the Settlement Category Molecular Formula: List here Form (physical state): List here Color: List here Boiling point: List here °C (List here °F) - lit. Potential Hazards/Toxicity Water reactive materials can react violently with water or atmospheric moisture to produce gas and heat. The risks associated with a specific chemical depend on its reactivity and the nature of the gaseous product (flammable, toxic, or both). The mutual production of flammable gas and heat can lead to spontaneous ignition or explosion. Typical gases produced are: H2, CH4, H2S, NH3, PH3, HCN, HF, HCl, HF, HI, SO2, and SO3. Prior to working with any water reactive chemicals you must identify which gas may be formed in case of exposure to water and learn the risks associated with this gas. The reaction rate of solid material (and therefore heat and gas generation) depends on the material's surface area. Therefore smaller particle size increases the hazards associated with these materials. Engineering Controls NOTE: Lab-specific information on engineering controls may be included in the Protocol/Procedure section. The following is a general plan for all water reactive materials: 1. Work under an inert atmosphere (e.g., argon, nitrogen) in a glove box. If work in a glove box is impractical or otherwise not indicated; be certain the material in not pyrophoric. 1. Work in a properly functioning certified chemical fume hood when handling water reactive materials. Work with the sash as low as possible. 2. Work away from any water sources or where there is the potential of water splash. 3. Use fresh, dry solvents. 4. Keep the material under inert atmosphere (e.g., nitrogen, argon) when not in use. Water Reactive Chemicals 2 1/2/2013 Personal Protective Equipment (PPE) NOTE: Lab-specific information on PPE selection may be included in the Protocol/Procedure section. Respiratory protection NOTE: Lab personnel intending to use/wear a respirator mask must be trained and fit-tested by EH&S. This is a regulatory requirement. Respirators should be used only under any of the following circumstances: As a last line of defense (i.e., after engineering and administrative controls have been exhausted). When Permissible Exposure Limit (PEL) has exceeded or when there is a possibility that PEL will be exceeded. Regulations require the use of a respirator. An employer requires the use of a respirator. There is potential for harmful exposure due to an atmospheric contaminant (in the absence of PEL) As PPE in the event of a chemical spill clean-up process Hand Protection Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. NOTE: Lab-specific and chemical-specific information on glove selection may be included in the Protocol/Procedure section. Refer to glove selection from the link below: For glove selection, go to: http://ehs.berkeley.edu/hs/63-laboratory-safety/94-glove-selection-andusage.html NOTE: Nomex-and-leather flight gloves over chemically resistant gloves are recommended in the UC Berkeley Office of Environmental Health and Safety document “Safe Use of Pyrophoric and Water Reactive Reagents”. http://www.ehs.berkeley.edu/hs/126-standard-operating-procedures-sop.html Eye Protection Tightly fitting safety goggles. Use face shield (8-inch minimum) when appropriate (not protected by fume hood sash for example). Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU) or ANSI Z87.1. Skin and Body Protection Long pants, closed-toed and closed-heeled shoes, cotton-based clothing/attire, and flame resistant lab coat must be worn for protecting against chemical hazards. NOTE: A Nomex lab coat is recommended in the UC Berkeley Office of Environmental Health and Safety document “Safe Use of Pyrophoric and Water Reactive Reagents”. http://www.ehs.berkeley.edu/hs/126-standard-operating-procedures-sop.html Hygiene Measures Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday. Water Reactive Chemicals 3 1/2/2013 First Aid Procedures Notify supervisor and EH&S immediately. Follow up with a call to 510-642-9090 to report the incident. If Inhaled Move person into fresh air. If not breathing, give artificial respiration. Consult a physician. In Case of Skin Contact Take off contaminated clothing immediately. Wash off with soap and plenty of water for 15 minutes. Take victim immediately to hospital. Consult a physician. In Case of Eye Contact Rinse thoroughly with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately. If Swallowed Do not induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. Special Handling and Storage Requirements NOTE: Lab-specific information on handling and storage may be included in the Protocol/Procedure section. Working Alone Certain extremely hazardous operations should not be performed if the PI or Lab Safety Contact(s) are not present. Never work alone with extremely hazardous materials/operations. See the Protocol/Procedure section below for specific prohibitions (if any) on working alone. NOTE: The UC Berkeley Office of Environmental Health and Safety document “Safe Use of Pyrophoric and Water Reactive Reagents” specifies not to work alone or during off hours, when there are few people around to help. http://www.ehs.berkeley.edu/hs/126-standard-operating-procedures-sop.html Precautions For Safe Handling Unless it is known otherwise, assume the material is pyrophoric. To be handled always in a glove box or under inert atmosphere. Design a quenching scheme for residual materials prior to using water reactive materials. Never use water to quench the material itself or a reaction where a water-reactive reagent is used. Begin quenching with a low reactivity quenching agent and slowly add more reactive quenching agents. For example, first quench residual sodium metal with isopropanol and then add ethanol to the mixture. Design your experiment to use the least amount of material possible to achieve the desired result. It is better to do multiple transfers of small volumes than attempt to handle larger quantities. Before transferring, make sure that the material is at room temperature. Avoid formation of dusts and aerosols Provide appropriate exhaust ventilation at places where dust is formed. Take measures to prevent the build-up of electrostatic charge. Keep away from sources of ignition – Open flames (e.g., Bunsen Burner) Eliminate or substitute a less hazardous material when possible. Verify your experimental set-up and procedure prior to use. Inform colleagues that this material will be used and where. Label the work area with a sign saying "Water Reactives Use Area". Water Reactive Chemicals 4 1/2/2013 Only use if the area is properly equipped with a certified eye wash/safety shower within ten seconds of travel. Never use water to extinguish fires caused by water reactive materials. NOTE: See a more details on safe handling in the UC Berkeley Office of Environmental Health and Safety documents “Safe Use of Pyrophoric and Water Reactive Reagents” and “Quenching of Pyrophoric Substances”. http://www.ehs.berkeley.edu/hs/126-standard-operating-procedures-sop.html Conditions for Safe Storage Never allow contact with water. Always handle inside a glove box. Over time, pressure may increase causing containers to burst. Keep container tightly closed in a cool, dry, well-ventilated place and protected from sunlight. Store and handle under inert gas (Noble gases such as Nitrogen, Argon etc.) Keep in a dry place (such as a desiccator or a dry box or glove box) free of moisture/humidity. Store away from heat sources and in a flame proof area Do not leave the container near a lab sink, emergency eyewash or safety shower. Store in a location, separated from acids, oxidizing and other incompatible materials. Use/purchase only amount that is needed in a reasonable amount of time. Use small quantities whenever possible. Store in a separate secondary container and label the material clearly. Minimize dust generation and accumulation. Hazard communication label on the container must read ‘Water Reactive’. W Never allow product to get in contact with water or water based compounds during storage. Do not leave the container on the bench top - even momentarily. Follow any substance-specific storage guidance provided in Safety Data Sheet documentation. Monitor your inventory closely to assure that you have tight control over your material. Wash hands and arms with soap and water after handling. Minimize dust generation and accumulation. At the end of each project, thoroughly inspect the area for residual reactive material. Spill and Accident Procedure Fire-fighting & Extinguishing media Suitable extinguishing media Carbon dioxide (CO2), Dry powder, and Class D fire extinguisher DO NOT use water Special Protective Equipment for Fire-Fighters Wear Self-Contained Breathing Apparatus (SCBA) for fire-fighting if necessary. Personal precautions Avoid dust formation. Ensure adequate ventilation. Remove all sources of ignition. Evacuate personnel to safe areas. Environmental precautions Water Reactive Chemicals 5 1/2/2013 Do not let product enter drains. Methods and materials for containment and cleaning up Pick-up and dispose of as hazardous waste without creating dust. Do not flush with water or bring in contact with moisture. Keep in suitable, tightly closed containers for disposal. Water Reactive Chemicals 6 1/2/2013 Chemical Spill Dial 911 Spill – Assess the extent of danger. Help contaminated or injured persons. Evacuate the spill area. Avoid breathing vapors. If possible, confine the spill to a small area using a spill kit or absorbent material. Keep others from entering contaminated area (e.g., use caution tape, barriers, etc.). Small (<1 L) – If you have training, you may assist in the clean-up effort. Use appropriate personal protective equipment and clean-up material for chemical spilled. Double bag spill waste in clear plastic bags, label and take to the next chemical waste pick-up. Large (>1 L) – Dial 911 and 510-642-9090 for assistance. Chemical Spill on Body or Clothes – Remove clothing and rinse body thoroughly in emergency shower for at least 15 minutes. Seek medical attention. Notify supervisor and EH&S immediately. Follow up with a call to 510-642-9090 to report the incident. Chemical Splash Into Eyes – Immediately rinse eyeball and inner surface of eyelid with water from the emergency eyewash station for 15 minutes by forcibly holding the eye open. Seek medical attention. Notify supervisor and EH&S immediately. Follow up with a call to 510-642-9090 to report the incident. Medical Emergency Dial 911 Life Threatening Emergency, After Hours, Weekends And Holidays – Dial 911 or go to the nearest emergency room. Note: All serious injuries must be reported to EH&S within 8 hours. Follow up with a call to 510-642-9090 to report the incident. Non-Life Threatening Emergency – Go to the Occupational Health Facility (Tang Health Center). After hours go to the nearest emergency room. Note: All serious injuries must be reported to EH&S within 8 hours. Follow up with a call to 510-642-9090 to report the incident. Needle stick/puncture exposure (as applicable to chemical handling procedure) – Wash the affected area with antiseptic soap and warm water for 15 minutes. For mucous membrane exposure, flush the affected area for 15 minutes using an eyewash station. Go to the Occupational Health Facility (Tang Health Center). After hours go to the nearest emergency room. Note: All needle stick/puncture exposures must be reported to EH&S within 8 hours. Follow up with a call to 510-642-9090 to report the incident. Decontamination/Waste Disposal Procedure NOTE: Lab-specific information on decontamination/waste disposal may be included in the Protocol/Procedure section. Wearing proper PPE, please decontaminate equipment and bench tops using procedures made in cooperation with site EHS&S. Please dispose of the water reactive and disposables contaminated with water reactives as hazardous waste. General hazardous waste disposal guidelines: Label Waste Label all containers with the label provided at http://ehs.berkeley.edu/hm/279-new-hazardous-wasteprogram-hwp.html. See the EH&S Fact Sheet, “Hazardous Waste Management” for general instructions on procedures for disposing of hazardous waste. Water Reactive Chemicals 7 1/2/2013 Store Waste Call EH&S for proper procedure to dispose of waste Store hazardous waste in closed containers, in secondary containment and in a designated location Double-bag dry waste using transparent bags Waste must be under the control of the person generating & disposing of it Dispose of Waste Dispose of regularly generated chemical waste within 6 months Call EH&S for questions Empty Containers o Dispose as hazardous waste if it once held extremely hazardous waste (irrespective of the container size) o Consult waste pick-up schedule Prepare for transport to pick-up location Check on-line waste tag Write date of pick-up on the waste tag Use secondary containment Dispose of regularly generated chemical waste within 6 months Call EH&S for questions Safety Data Sheet (SDS) Location SDS can be accessed online at http://ucmsds.com Water Reactive Chemicals 8 1/2/2013 Documentation of Training (signature of all users is required) Prior to conducting any work with water reactive chemicals, designated personnel must provide training to his/her laboratory personnel specific to the hazards involved in working with the substance(s), work area decontamination, and emergency procedures. The Principal Investigator must provide his/her laboratory personnel with a copy of this SOP and a copy of the SDS provided by the manufacturer. I have read and understand the content of this SOP: Name Signature Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Water Reactive Chemicals 9 SOP Template developed by The UC Center for Laboratory Safety Initials Identification Date Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Date: 1/2/2013 Appendix A (from Case Settlement Appendix 2) Water Reactive Chemicals Aluminum alkyl halides Aluminum alkyl hydrides Aluminum alkyls Aluminum borohydride or Aluminum borohydride in devices Aluminum Carbide Aluminum ferrosilicon powder Aluminum hydride Aluminum phosphide Aluminum powder, uncoated Aluminum silicon powder, Uncoated < Barium ~. I Boron trifluoride dimethyl etherate Calcium Calcium carbide Calcium cyanamide with more than 0.1percent of calcium carbide Calcium hydride Calcium manganese silicon Calcium phosphide Calcium silicide Cells, containing sodium Cerium, turnings or gritty powder Cesium or Caesium Diethylzinc Dimethylzinc Water Reactive Chemicals 10 SOP Template developed by The UC Center for Laboratory Safety Date: 1/2/2013 Ethyldichlorosilane Ferrosilicon, with 30 percent or more but less than 90 percent silicon Hexyllithium Lithium Lithium alkyls Lithium aluminum hydride Lithium aluminum hydride, ethereal Lithium borohydride Lithium ferrosilicon Lithium hydride Lithium hydride, fused solid Lithium nitride Lithium silicon Magnesium alkyls Magnesium aluminum phosphide Magnesium granules, coated, particle size not less than 149 microns Magnesium hydride Magnesium phosphide Magnesium silicide Magnesium, powder or Magnesium alloys, powder Maneb or Maneb preparations with not less than 60 percent maneb Methyl magnesium bromide, in ethyl ether Methyldichlorosilane Phosphorus pentasulfide, free from yellow or white phosphorus Potassium Potassium borohydride Potassium phosphide Potassium sodium alloys Water Reactive Chemicals 11 SOP Template developed by The UC Center for Laboratory Safety Date: 1/2/2013 Potassium, metal alloys Rubidium Sodium Sodium aluminum hydride Sodium borohydride Sodium hydride Sodium phosphide Starmic phosphide Strontium phosphide Trichlorosilane Zinc ashes Zinc phosphide Zinc powder or Zinc dust [Water Reactive Chemicals] 12 SOP Template developed by The UC Center for Laboratory Safety Date: 1/2/2013