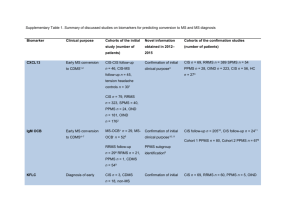
Supplementary Table 1. Evidence for glutamatergic system
advertisement

Supplementary Table 1. Evidence for glutamatergic system dysfunction in MS Sample Method Finding Reference CSF (RRMS) HPLC ↑ Stover et al.1 CSF (RRMS) HPLC ↑ Sarchielli et al.2 CSF Proteomics ↑ Pieragostino et al.3 CP, AP, WM (RRMS, SPMS, PPMS) Normal WM (RRMS, SPMS, PPMS) Normal WM and GM (all types of MS) WM (SP-MS) MRS 3T No change in CP ↑normal-appearing WM, AP ↑ Srinivasan et al.4 ↑ Baranzini et al. 6 MRS ↓ Mac Millan et al. 7 GM (RRMS) MRI ↓Small Muhlert et al.8 CSF (unspecified MS) HPLC ↓ Qureshi & Baig9 IHC ↑Glutaminase Werner et al.10 IHC ↓GS and GDH Werner et al.10 IHC ↑ GS and GDH Newcombe et al.11 Proteomics ↑GDH Han et al.12 OLG of WM AP and CP (PPMS) OLG of WM AP IHC ↓EAAT1 and GLT1 Werner et al.10 IHC ↓EAAT1 and GLT1 Pitt et al.13 Optic nerve (RRMS, SPMS, PPMS) Cortical lesions (RRMS, SPMS) Spinal cord (SPMS, PPMS) IHC, WB, qPCR IHC ↑EAAT1 and GLT1 ↓EAAT1 and GLT1 Vallejo-Illarramendi et al.14 Vercellino et al.15 IHC microarray WB IHC ↑ EAAT1 Lieury et al.16 ↑EAAC1 Newcombe et al.11 WB, IHC, qPCR ↑cystine/Glu antiporter Pampliega et al.17 Uptake ↑ in glial vesicles Vallejo-Illarramendi et al.14 Non active WM, CP and AP (SPMS, PPMS) OLG AP IHC Geurts et al.18 IHC ↑mGluR1 (dystrophic axon); mGluR5 and mGluR2/3 (astrog) ↑AMPAR GluR1 OLG CP IHC ↓ AMPAR GluR1 Microglia AP Astroglia and dystrophic axon Endothelial cells and dystrophic axon Cerebellum (SPMS, PPMS, RRMS) CAP and AP (SPMS, PPMS, RRMS) CP (RRMS) IHC IHC ↑ AMPAR GluR2 ↑mGluR1,mGluR2/3, mGluR5 IHC ↑ mGluR5–7 IHC ↓ mGluR1 and↑ mGluR5 Fazio et al.19 Proteomics ↑ mGluR2 in CAP ↑ mGluR3 in AP Han et al.12 WB ↑ AMPAR GluR2 Zhai et al.20 Glutamate level MRI and MRS MRI Vrenken et al.5 Glutamate-metabolizing enzymes Macrophages/microglia WM (PPMS) OLG of WM AP and CP Glutamate (PPMS) degradation Microglia and astroglia AP CP and AP (SPMS, PPMS) Glutamate transporters/uptake Glutamate synthesis Glutamate transporters Microglia and astroglia AP Spinal cord leukocytes and Optic nerve (RRMS, SPMS) Optic nerve (PPMS, Glutamate uptake SPMS RRMS) Glutamate receptor (GluR) expression Newcombe et al.11 Abbreviations: AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; AP, active plaque; CAP, chronic active plaque; CP, chronic plaque; CSF, cerebrospinal fluid; EAAC1, excitatory amino-acid carrier 1; GDH, glu dehydrogenase; EAAT1, glutamate aspartate transporter; GM, grey matter; GS, glutamine synthetase; HPLC, high-performance liquid chromatography; IHC, immunohistochemistry; mGluR, metabotropic glutamate receptor; MS, multiple sclerosis; MRS, magnetic resonance spectroscopy; NMDAR, N-methyl-D-aspartate receptor; OLG, oligodendrocyte; PP, primary progressive; qPCR, quantitative PCR; RR, relapsing–remitting; SP-secondary progressive; WB, western blot; WM, white matter. 1. Stover, J. F. et al. Neurotransmitters in cerebrospinal fluid reflect pathological activity. Eur. J. Clin. Invest. 27, 1038–1043 (1997). 2. Sarchielli, P., Greco, L., Floridi, A., Floridi, A. & Gallai, V. Excitatory amino acids and multiple sclerosis: evidence from cerebrospinal fluid. Arch. Neurol. 60, 1082–1088 (2003). 3. Pieragostino, D. et al. An integrated metabolomics approach for the research of new cerebrospinal fluid biomarkers of multiple sclerosis. Mol. Biosyst. 11, 1563–1572 (2015). 4. Srinivasan, R., Sailasuta, N., Hurd, R., Nelson, S. & Pelletier, D. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain 128, 1016–1025 (2005). 5. Vrenken, H. et al. MR spectroscopic evidence for glial increase but not for neuro-axonal damage in MS normal-appearing white matter. Magn. Reson. Med. 53, 256–266 (2005). 6. Baranzini, S. E. et al. Genetic variation influences glutamate concentrations in brains of patients with multiple sclerosis. Brain 133, 2603-2611 (2010). 7. MacMillan, E. L. et al. Progressive multiple sclerosis exhibits decreasing glutamate and glutamine over two years. Mult. Scler. (2015). doi:10.1177/1352458515586086 8. Muhlert, N. et al. Memory in multiple sclerosis is linked to glutamate concentration in grey matter regions. J. Neurol. Neurosurg. Psychiatry 85, 834–840 (2014). 9. Qureshi, G. A. & Baig, M. S. Quantitation of free amino acids in biological samples by high-performance liquid chromatography. Application of the method in evaluating amino acid levels in cerebrospinal fluid and plasma of patients with multiple sclerosis. J. Chromatogr. 459, 237–244 (1988). 10. Werner, P., Pitt, D. & Raine, C. S. Multiple sclerosis: Altered glutamate homeostasis in lesions correlates with oligodendrocyre and axonal damage. Ann. Neurol. 50, 169–180 (2001). 11. Newcombe, J. et al. Glutamate receptor expression in multiple sclerosis lesions. Brain Pathol. 18, 52–61 (2008). 12. Han, M. H. et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature 451, 10761081 (2008). 13. Pitt, D., Nagelmeier, I. E., Wilson, H. C. & Raine, C. S. Glutamate uptake by oligodendrocytes: Implications for excitotoxicity in multiple sclerosis. Neurology 61, 1113–1120 (2003). 14. Vallejo-Illarramendi, A., Domercq, M., Pérez-Cerdá, F., Ravid, R. & Matute, C. Increased expression and function of glutamate transporters in multiple sclerosis. Neurobiol. Dis. 21, 154–164 (2006). 15. Vercellino, M. et al. Altered glutamate reuptake in relapsing-remitting and secondary progressive multiple sclerosis cortex: correlation with microglia infiltration, demyelination, and neuronal and synaptic damage. J. Neuropathol. Exp. Neurol. 66, 723-729 (2007). 16. Lieury, A. et al. Tissue Remodeling in Periplaque Regions of Multiple Sclerosis Spinal Cord Lesions. Glia 62, 1645– 1658 (2014). 17. Pampliega, O. et al. Increased expression of cystine/glutamate antiporter in multiple sclerosis. J. Neuroinflammation 8, 63 (2011). 18. Geurts, J. J. G. et al. Altered expression patterns of group I and II metabotropic glutamate receptors in multiple sclerosis. Brain 126, 1755–1766 (2003). 19. Fazio, F. et al. Switch in the expression of mGlu1 and mGlu5 metabotropic glutamate receptors in the cerebellum of mice developing experimental autoimmune encephalomyelitis and in autoptic cerebellar samples from patients with multiple sclerosis. Neuropharmacology 55, 491–499 (2008). 20. Zhai, D. et al. Blocking GluR2-GAPDH ameliorates experimental autoimmune encephalomyelitis. Ann. Clin. Transl. Neurol. 2, 388–400 (2015).