pubdoc_11_1759_1660
advertisement

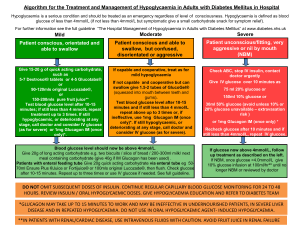
Lec: 7 Dr. Mohammed Alhamdany Incidental adrenal mass It is not uncommon for a mass in the adrenal gland to be identified on a CT or MRI scan of the abdomen that has been performed for another indication. Such lesions are known as adrenal ‘incidentalomas’. They are present in up to 10% of adults and the prevalence increases with age. Eighty-five per cent of adrenal incidentalomas are non-functioning adrenal adenomas. The remainder includes functional tumours of the adrenal cortex (secreting cortisol, aldosterone or androgens), phaeochromocytomas, primary and secondary carcinomas. There are two key questions to be resolved: is the lesion secreting hormones, and is it benign or malignant? clinical signs and symptoms: Including feature of excess glucocorticoids, mineralocorticoids, catecholamines, and, in women, androgens should be sought. Investigations Include a dexamethasone suppression test, urine or plasma metanephrines and, in virilised women, measurement of serum testosterone, DHEA. Patients with hypertension should be investigated for mineralocorticoid excess. CT and MRI are equally effective in assessing the malignant potential of an adrenal mass, using the following parameters: 1- Size: The larger the lesion, the greater the malignant potential. 2- Configuration: Homogeneous and smooth lesions are more likely to be benign. 3- Presence of lipid: Adenomas are usually lipid-rich, resulting in an attenuation of below 10 Hounsfield Units (HU), so low density lesion tend to be benign. 4- Enhancement: malignant lesion tend to take more contrast (enhanced). Management Functional lesions and tumours of more than 4 cm in diameter should be considered for removal by adrenal surgery. 1 THE ENDOCRINE PANCREAS AND GASTROINTESTINAL TRACT These include: a- Hormonal excess: 1- Insulinoma. 2- Gastrinoma (Zollinger–Ellison syndrome). 3- Carcinoid syndrome (secretion of 5-HT). 4- Glucagonoma. 5- VIPoma. 6- Somatostatinoma. b- Hormone deficiency Diabetes mellitus. c- Hormone resistance Insulin resistance syndromes (e.g. type 2 diabetes mellitus) Presenting problems in endocrine pancreas disease Spontaneous hypoglycaemia: Hypoglycaemia most commonly occurs as a side-effect of treatment with insulin or sulphonylurea drugs in people with diabetes mellitus. In non-diabetic individuals, symptomatic hypoglycaemia is rare, but it is not uncommon to detect venous blood glucose concentrations below 3.0 mmol/L in asymptomatic patients. a hypoglycaemic disorder should only be diagnosed if all three conditions of Whipple’s triad are met. Symptoms occurring while fasting (such as before breakfast) or following exercise are much more likely to be representative of pathological hypoglycaemia than those which develop after food (post-prandial or ‘reactive’ symptoms). Hypoglycaemia should be considered in all comatose patients. Investigations Does the patient have a hypoglycaemic disorder? In acute presentation: Patients who present acutely with confusion, coma or convulsions should be tested for hypoglycaemia at the bedside with a capillary blood sample and an automated meter. At the same time, a sample should be taken for later measurement, if necessary, of alcohol, insulin, C-peptide, cortisol and sulphonylurea levels. In episodic presentation: Patients who attend the outpatient clinic with episodic symptoms suggestive of hypoglycaemia present a more challenging problem. The main diagnostic test is the prolonged (72-hour) fast. If symptoms of hypoglycaemia develop during the fast, then blood samples should be taken to confirm hypoglycaemia and for later measurement of insulin and C-peptide. Hypoglycaemia is then corrected with oral or intravenous glucose and Whipple’s triad completed by confirmation of the resolution of symptoms. 2 What is the cause of the hypoglycaemia? In the acute setting, the underlying diagnosis is often obvious. In non-diabetic individuals, alcohol excess is the important cause of hypoglycemia. Hypoglycaemia is one of many metabolic derangements which occur in patients with hepatic failure, renal failure, sepsis or malaria. insulinomas in the pancreas are small (< 5 mm diameter) but can be identified by CT, MRI or ultrasound (endoscopic or laparoscopic). Imaging should include the liver since around 10% of insulinomas are malignant. Rarely, large nonpancreatic tumours, such as sarcomas, may cause recurrent hypoglycaemia because of their ability to produce excess pro-insulin-like growth factor-2 (proIGF-2). Management Treatment of acute hypoglycaemia should be initiated as soon as laboratory blood samples have been taken, and should not be deferred until formal laboratory confirmation has been obtained. 1- Intravenous dextrose (5% or 10%): is effective in the short term in the obtunded patient, and should be followed on recovery with oral unrefined carbohydrate (starch). Continuous dextrose infusion may be necessary, especially in sulphonylurea poisoning. 2- Intramuscular glucagon (1 mg): stimulates hepatic glucose release, but is ineffective in patients with depleted glycogen reserves, such as in alcohol excess or liver disease. 3- for Chronic recurrent hypoglycaemia: in insulin-secreting tumours can be treated by regular consumption of oral carbohydrate combined with agents that inhibit insulin secretion (diazoxide or somatostatin analogues). Insulinomas are resected when benign. 3 Gastroenteropancreatic neuro-endocrine tumours: Neuro-endocrine tumours (NETs) are a heterogeneous group derived from neuro-endocrine cells in many organs, including: 1- the gastrointestinal tract (insulinoma). 2- lung((small-cell carcinoma of the lung). 3- adrenals (phaeochromocytoma). 4- thyroid (medullary carcinoma). Most NETs occur sporadically, but a proportion are associated with genetic cancer syndromes, such as MEN 1, MEN 2 and neurofibromatosis type 1. The majority of gastroenteropancreatic NETs behave in an intermediate manner, with relatively slow growth but a propensity to invade and metastasise to remote organs, especially the liver. Clinical features Patients with gastroenteropancreatic NETs often have a history of abdominal pain over many years prior to diagnosis and usually present with local mass effects, such as small-bowel obstruction, appendicitis, and pain from hepatic metastases. Carcinoid tumor: The classic ‘carcinoid syndrome’ occurs when vasoactive hormones ( such as serotonin and bradykinin) reach the systemic circulation. In the case of gastrointestinal carcinoids, this invariably means that the tumour has metastasised to the liver or there are peritoneal deposits, which allow secreted hormones to gain access to the systemic circulation; hormones secreted by the primary tumour into the portal vein are metabolised and inactivated in the liver. It characterized by the following clinical feature: 1- Episodic flushing, wheezing and diarrhea. 2- Facial telangiectasia. 3- Cardiac involvement (tricuspid regurgitation, pulmonary stenosis, right ventricular endocardial plaques) leading to heart failure. The clinical feature of each pancreatic endocrine tumor: 1- Gastrinoma secretes Gastrin and cause Peptic ulcer and steatorrhoea (Zollinger–Ellison syndrome). 2- Insulinoma secretes Insulin and cause recurrent hypoglycemia. 3- VIPoma secretes vasoactive intestinal peptide (VIP) and cause Watery diarrhoea and hypokalaemia. 4- Glucagonoma secretes Glucagon and cause Diabetes mellitus, and necrolytic migratory erythema. 5- Somatostatinoma secretes Somatostatin and cause Diabetes mellitus and steatorrhoea. Investigations A combination of imaging with ultrasound, CT, MRI and/or radio-labelled somatostatin analogue will usually identify the primary tumour and allow staging, which is crucial for determining prognosis. 4 Biopsy of the primary tumour or a metastatic deposit is required to confirm the histological type. Carcinoid syndrome is confirmed by measuring elevated concentrations of 5-hydroxyindoleacetic acid (5-HIAA), a metabolite of serotonin, in a 24 hour urine collection. Plasma chromogranin A can be measured in a fasting blood sample, along with the hormones listed above. Management Treatment of solitary tumours is by surgical resection. If metastatic or multifocal primary disease is present, then surgery is usually not indicated, unless there is a complication such as gastrointestinal obstruction. Diazoxide can reduce insulin secretion in insulinomas, and high doses of proton pump inhibitors suppress acid production in gastrinomas. Somatostatin analogues are effective in reducing the symptoms of carcinoid syndrome and of excess glucagon and vasoactive intestinal peptide (VIP) production. The slow-growing nature of NETs means that conventional cancer therapies, such as chemotherapy and radiotherapy, have limited efficacy. Other treatments, such as interferon, targeted radionuclide therapy with 131IMIBG and radio-labelled somatostatin analogues (which may be taken up by NET metastases) and resection/embolisation of hepatic metastases, may have a role in the palliation of symptoms but there is little evidence that they prolong life. With best regard 5