Royal College of Midwives Update Update on Sodium Valproate
advertisement

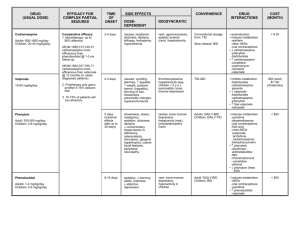
Royal College of Midwives Update Update on Sodium Valproate update by Jacque Gerrard 9/2/15 Dear HOMS, As you know there has been a recent MHRA led EU review of sodium valproate use and pregnancy. This review has concluded that the guidelines on the use of valproate in girls and women of childbearing age need to be strengthened through updates to patient information leaflets and healthcare professional guidance. The Commission on Human Medicine supports the introduction of the measures, and has asked the MHRA to undertake further work with stakeholders on an ‘acknowledgement of risk’ form. To support and supplement the MHRA material, DH England are working on measures to improve awareness which includes working with IT suppliers to introduce a ‘red-flag’ warning systems to GP and community pharmacy IT systems. The will also provide updated information through NHS Choices and bulletins produced by DH England and NHS England. For further information see attached Central Alerting System notice from NHS England. This has been circulated across England. /w EPDw UJMTE0O 6E6E4C58 0 0 HomeHelpLogin <span> <b>Javascript is disabled because of the browser settings on this machine. So, a few features might not be available.</b> </span> View Alert Originator: MHRA Dear Doctor Letter From: Dr June Raine, Director, Vigilance and Risk Management of Medicines Issue date: 21-Jan-2015 14:51:41 Action by recipients: NHS England Area Teams NHS Foundation Trusts (England) - Medical Director NHS Foundation Trusts (England) - Chief Executive NHS Trusts (England) - Medical Director Territorial CMOs in Ireland, Scotland & Wales Regional Directors of Public Health CMO Urgent Messages - Recipients on Public Health Link NHS Trusts (England) - Chief Executive Information to recipients: CMO Urgent Messages - Non-NHS Recipients on Public Health Link Social Care Providers (registered with CAS) Director of Public Health Clinical Commissioning Groups Action category: Non urgent (cascade within 48 hours) Royal College of Midwives Update Title: Medicines related to valproate: risk of abnormal pregnancy outcomes Broadcast content: This letter is to inform you of important new information and strengthened warnings related to safety of medicines related to valproate (sodium valproate, valproic acid [brand leader: Epilim] and valproate semisodium [brand leader: Depakote]), following completion of a Europe-wide review. Please see the attached pdf documents. Additional information: Please cascade to all primary care providers including GPs, dispensing GPs and all pharmacists (community and hospital pharmacists) Alert reference: DDL_valproate Attachments: Valproate-risk of abnormal pregnancy outcomes-letter to healthcare professionals Jan 2015.pdf Valproate guide for healthcare professionals Jan 2015.pdf Valproate booklet for patients Jan 2015.pdf Cascadeto: #GP# #ACCIDENTEMERGENCY# #COMMUNITYPHARMACISTS# #DISPENSING GP# Contact the CAS helpdesk Telephone: 020 3080 6747 Email: safetyalerts@dh.gsi.gov.uk