etc2462-sm-0001-SuppData-S1
advertisement

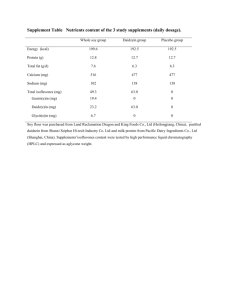
SUPPORTING INFORMATION For PHYTOESTROGENS IN THE ENVIRONMENT: II. MICROBIOLOGICAL DEGRADATION OF PHYTOESTROGENS AND THE RESPONSE OF FATHEAD MINNOWS TO DEGRADATE EXPOSURE MEGAN M. KELLY†, NATHAN T. FLEISCHHACKER‡, DANIEL C. REARICK§, WILLIAM A. ARNOLD†‡, HEIKO L. SCHOENFUSS§, AND PAIGE J. NOVAK†‡* † Water Resources Science Graduate Program, University of Minnesota, St. Paul, Minnesota, USA ‡ Department of Civil Engineering, University of Minnesota, St. Paul, Minnesota, USA § Aquatic Toxicology Laboratory, St. Cloud State University, St. Cloud, Minnesota, USA 1 METHODS Table S1. Nitrification reactor media Concentration Chemical Formula mg/L Sodium Phosphate Na2HPO4-7H2O Potassium KH2PO4 3,000 83.3 Phosphate Magnesium Sulphate MgSO4-7H20 80 Calcium Chloride CaCl2 75 Sodium Bicarbonate NaHCO3 1.5 Ferric Chloride FeCl3-6H2O 0.8 Copper Sulphate CuSO4 0.2 EDTA Na3EDTA-4H2O 1 Cobalt Chloride CoCl2-6H2O 2.0 x 10-4 Zinc Sulphate ZnSO4-7H2O 0.1 Sodium Molybdate Na2MoO4-2H2O 0.1 Manganese Chloride MnCl2-2H2O 2 Ammonium Sulphate (NH4)2SO4 1,000 2 Table S2. Confirmatory water chemistry for the larval minnow phytoestrogen exposure experiment (mean ± st. err.; n=2) averaged over the exposure period. Treatment Genistein Daidzein Formononetin Lake Water Control Non-detect 40 ± 57 ng/L 25 ± 35 ng/L Degraded genistein 30 ± 42 ng/L 115 ± 163 ng/L Non-detect Degraded daidzein Non-detect 140 ± 198 ng/L 30 ± 42 ng/L Degraded mixture 70 ± 0 ng/L 90 ± 127 ng/L Non-detect Note: the daidzein detection in all the treatments was thought to be a co-eluting compound as a result of biological activity in the system. A similar compound was detected in similar studies fed parent (non-degraded) phytoestrogens [S1]. In addition, similar studies showed that daidzein exposure did not cause a measureable response larval minnows [S1]; therefore, the presence of either daidzein (unlikely) or a co-eluting compound in these samples (likely) was deemed to be unimportant with respect to larval response. 3 Table S3. Concentrations of phytoestrogens measured (mean ± standard deviation; n=5) in the ethanol blank [further diluted with well water 1:588,000 prior to minnow exposure], the bioreactor feed, and in the bioreactor effluent [further diluted with well water 1:400 prior to minnow exposure] in the experiment to test the effect of adult minnow exposure to the biodegraded phytoestrogens. Treatment Ethanol blank Bioreactor feed Genistein Daidzein Formononetin 17.6 ± 2.4 ng/L Non-detect 8.8 ± 0.7 ng/L 108,810 ± 8,210 ng/L 65,260 ± 5,640 ng/L 163,050 ± 6,620 ng/L 34.3 ± 33.6 ng/L Non-detect 8.5 ± 0.8 ng/L Bioreactor effluent Optical density (OD), suspended solids (SS), volatile suspended solids (VSS), and dissolved organic carbon (DOC) analysis. OD was measured using a Beckman DU 530 UV/VIS Spectrophotometer (Fullerton, CA) at a wavelength of 600 nm. Well-mixed samples (2 mL) were placed in cuvettes (Life Sciences, Foster City, CA) and measured three times; the average value was recorded. SS and VSS were measured according to Standard Method 2540D and 2540E [S2], respectively. Samples (20 mL) were analyzed for DOC by filtering them through a GFF, acidifying the filtrate to pH 2 with 5 M H 2SO4, purging inorganic carbon with N2 gas, then analyzing the residual carbon (assumed to be organic) with a Sievers 900 Portable TOC Analyzer (General Electric, Fairfield, CT). 4 Ion analysis. Nitrate and nitrite concentrations were determined using a Metrohm (Riverview, FL, USA) 761 ion chromatograph using a Metrohm 766 sample processor and IC Net software. The eluent solution consisted of 1mM NaHCO3 and 32 mM Na2CO3. Regenerant was a 0.2 mM sulfuric acid solution. A combined external calibration curve for nitrate and nitrite in Milli-Q was used to quantify nitrate and nitrite. 5 RESULTS 120 Concentration (g/L) 100 80 60 40 20 0 0 10 20 30 50 40 60 Time (hrs) Figure S1. Degradation of genistein (red) and daidzein (blue) in Mississippi River water collected on June 26, 2013, incubated at 20° C. Long-dash lines represent fits to the Gompertz equation, short-dash lines to zero-order kinetics, and dash-dot lines to firstorder kinetics. Empty circles represent controls. 6 120 A Concentration (g/L 100 10 B 8 80 6 60 4 40 2 20 0 0 2.0 C 0.8 D 1.8 Concentration (g/L 1.6 0.6 1.4 1.2 1.0 0.4 0.8 0.6 0.2 0.4 0.2 0.0 0 20 40 60 80 0.0 100 Time (hrs) 0 20 40 60 80 100 Time (hrs) Figure S2. Degradation of genistein with an initial concentration of 50 μg/L (A), 10 μg/L (B), 1 μg/L (C), and 0.5 μg/L (D) in Mississippi River water collected on June 26, 2013, incubated at 20° C. Long-dash lines represent fits to the Gompertz equation, short-dash lines to zero-order kinetics, and dash-dot lines to first-order kinetics. Empty circles represent controls. 7 70 12 A Concentration (g/L) 60 10 50 8 40 6 30 4 20 2 10 0 0 1.4 0.6 C 1.2 Concentration (g/L) B D 0.5 1.0 0.4 0.8 0.3 0.6 0.2 0.4 0.2 0.1 0.0 0.0 0 20 40 60 80 100 Time (hrs) 0 20 40 60 80 100 Time (hrs) Figure S3. Degradation of daidzein with an initial concentration of 50 μg/L (A), 10 μg/L (B), 1 μg/L (C), and 0.5 μg/L (D) in Mississippi River water collected on June 26, 2013, incubated at 20° C. Long-dash lines represent fits to the Gompertz equation, short-dash lines to zero-order kinetics, and dash-dot lines to first-order kinetics. Empty circles represent controls. 8 50 Inhibitor added 40 mg N/L 30 20 10 0 0 10 20 30 40 50 60 Time (hrs) Figure S4. Concentrations of nitrite (black), nitrate (white), and ammonia (grey) in an enriched culture of nitrifying organisms before and after the addition of allylthiourea. 9 Figure S5. Percent larval fathead minnow survival during a 21-day exposure to the degradation compounds singly and in mixture. Initial cohorts were established using 75 larvae per treatment. Survival represents number of individuals remaining after behavioral testing. 10 Figure S6. Larval fathead minnow escape performance following 21-day exposure to degraded phytoestrogen compounds. C-start escape performance was quantified using. (A) body length (mm); (B) mean latency (ms) from stimulus to response; (C) mean escape velocity relative to body length (BL/ms); (D) mean total escape performance (BL/ms) defined as (distance travelled/BL)/(40+latency) (n = 30 for all treatments). 11 Figure S7. Mean ± standard error plasma vitellogenin concentrations (g/mL) in male (A) and female (B) fathead minnows exposed for 21- days to the degraded phytoestrogen mixture (Daidzein, Genistein, and Formononetin 1,000 ng/L per compound). Severity of hepatocyte vacuole presence for male (C) and female (D) minnows. Testis (E) and ovarian 12 (F) maturity. Sample size for each treatment is listed in each column. Sample sizes differed between endpoints and treatments due to insufficient tissue or analysis failure. 13 Figure S8. Body condition factor (A-male; B-female), hepatosomatic idnex (C,D) and gonadosomatic index (E,F) for fathead minnows exposed for 21 days to the degradation 14 mixture. Sample size for each treatment (and all three endpoints) is listed in each column of (A) and (B), respectively. 15 Figure S9. Mean ± standard error expression of secondary sex characteristics (A) and total aggression index (B) for male fathead minnows exposed 21-days to the degradation mixture. Sample size for each treatment is listed in each column REFERENCES S1. Rearick DC, Fleischhacker NT, Kelly MM, Arnold, WA, Novak PJ, Schoenfuss HL. Environmental occurrence of phytoestrogens and exposure effects on fathead minnows. Environ. Toxicol. Chem. submitted. S2. Yi T, Harper W. 2007. The link between nitrification and biotransformation of 17αethinylestradiol. Environ. Sci. Technol. 41:4311-6. 16