Solutions Review Determine the concentration of a 250 mL solution
advertisement

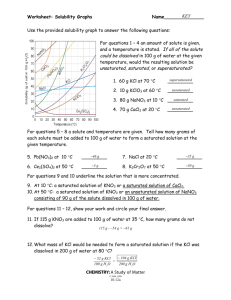
Solutions Review 1. Determine the concentration of a 250 mL solution containing 0.14 moles of solute. 2. Determine the concentration of a 3.5 L solution containing 80.0 g of NaOH, molar mass = 39.997 g/mol. 3. How many moles of solute are in a 1.25 L solution that is 0.15 M. 4. A 8.0 M solution containing 1.85 moles of solute will occupy what volume? 5. Find the volume, in mL, of a 3.5 M solution containing 0.045 moles of solute. 6. List two ways to make a saturated solution unsaturated. 7. Determine the concentration of a 575 mL solution that was made by diluting 100.0 mL of 5.0 M solution. 8. To what volume, in mL, must a 12.0 M, 0.500 L solution be diluted to in order to create a 2.5 M solution? 9. Find the solubility of the following compounds at 80°C. a. KCl b. NH4Cl c. KClO3 10. Find the min temp to dissolve100 g of NaNO3 11. How much more KCl can be dissolved at 80°C than at 30°C? 12. If 120 g of KClO3 is added to 100 g of water at 70°C, how much will not dissolve? 13. A 750 mL, 3.0 M must be diluted with what volume of solvent to create a 2.25 M solution? 14. A saturated solution of KNO3 initially at 30°C is heated to 65°C, how much more KNO3 can be dissolved. 15. Determine if the following solutions are saturated or unsaturated a. A solution containing 100 g of water and 50 g of NH4Cl at 50°C b. 30 g of NaNO3 in 100 g of water at 45°C c. 50 g of NaCl in 100 g of water at 80°C Solutions Review 1. Determine the concentration of a 250 mL solution containing 0.14 moles of solute. 0.14 / 0.250 = 0.56 M 2. Determine the concentration of a 3.5 L solution containing 80.0 g of NaOH, molar mass = 39.997 g/mol. 80.0 / 39.997 = 2.00 moles 2.00 / 3.5 = 0.57 M 3. How many moles of solute are in a 1.25 L solution that is 0.15 M. 0.15 = n / 1.25 n = 0.19 moles 4. A 8.0 M solution containing 1.85 moles of solute will occupy what volume? 8.0 = 1.85 / V V = 0.23 L or 230 mL 5. Find the volume, in mL, of a 3.5 M solution containing 0.045 moles of solute. 3.5 = 0.045 / V V = 0.013 L = 13 mL 6. List two ways to make a saturated solution unsaturated. Heat it up or add more solvent 7. Determine the concentration of a 575 mL solution that was made by diluting 100.0 mL of 5.0 M solution. M1 (575) = (5.0) (100) M1 = 0.87 M 8. To what volume, in mL, must a 12.0 M, 0.500 L solution be diluted to in order to create a 2.5 M solution? (12.0) (500) = (2.5) V2 V2 = 2,400 mL 9. Find the solubility of the following compounds at 80°C. a. KCl 50 g b. NH4Cl 65 g c. KClO3 40 g 10. Find the min temp to dissolve100 g of NaNO3 35 °C 11. How much more KCl can be dissolved at 80°C than at 30°C? (50 – 35) = 15 g 12. If 120 g of KClO3 is added to 100 g of water at 70°C, how much will not dissolve? (120 – 33) = 87 g 13. A 750 mL, 3.0 M must be diluted with what volume of solvent to create a 2.25 M solution? (3.0) (750) = (2.25) V2 V2 = 1000 mL 1000 – 750 = 250 Solution must be diluted with 250 mL of solvent 14. A saturated solution of KNO3 initially at 30°C is heated to 65°C, how much more KNO3 can be dissolved. (115 – 45) = 70 g 15. Determine if the following solutions are saturated or unsaturated a. A solution containing 100 g of water and 50 g of NH4Cl at 50°C Sat b. 30 g of NaNO3 in 100 g of water at 45°C Unsaturated c. 50 g of NaCl in 100 g of water at 80°C Saturated