1 st Nine Weeks Test Review
advertisement

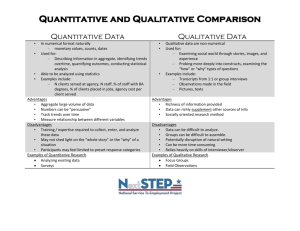
Name _________________________________ Date_________________ Hour____________ Table #________ 1st Nine Weeks Test Review Define the following: Independent Variable: Dependent Variable: Constant: Control: Gas: Liquid: Solid: Density: Qualitative Change: Quantitative Change: Atom: Molecule: Physical Change: Chemical Change: Potential Energy: Kinetic Energy: Thermal Energy: Chemical Energy: Name _________________________________ Date_________________ Hour____________ Table #________ Student Notes: I investigated yeast, tiny organisms that give off carbon dioxide gas as they grow. In two bottles, I put 2 mL of yeast, 5 mL of sugar, and water. In Bottle A, I used 250 mL of cold water (20◦C). In Bottle B, I used 250 mL of warm water (40◦C). I attached a balloon to each bottle. After five minutes, I observed bubbles forming on the surface inside the bottles, and the balloons on both bottles expanded. The balloon on Bottle B became twice as large as the balloon on Bottle A. Use the student notes above to match the correct information from Column 2 with each item in Column 1. Write the letter of the correct answer on the line at the left of the numbers in column 1. Column 1 Column 2 A. If the water temperature is increased, then the yeast will give off more gas, because the heat speeds ______1. Problem or Question things up. ______2. Hypothesis ______3. Materials B. After five minutes, Balloons A and B expanded C. Yeast gives off more gas at higher temperatures D. What factors help yeast grow? ______4. Procedure ______5. Observations ______6. Analysis ______7. Conclusion E. Balloon B became bigger than Balloon A, so that means that the yeast in Balloon B gave off more gas. F. Combine the yeast, sugar and water in the bottle. Put a balloon on the bottle G. 4 mL of yeast, 10 mL of sugar, 250 mL of warm water, 250 mL of cool water. 8. For the following examples, identify the INDEPENDENT VARIABLE (manipulated), the DEPENDENT VARIABLE (responding) and THREE CONSTANTS (controlled variables). Does the flavor of gum affect how big a bubble Suzie can blow? Independent: Dependent: Constant: Constant: Constant: Name _________________________________ Date_________________ Hour____________ Table #________ It All Matters! Properties of Matter A. Use the following list of words to complete the passage about matter and changes of properties in matter. A word or words may be used more than once or not at all. chemical chemical change chemical equation chemical reaction mass physical physical change Products reactants volume Matter is anything that has both (9) _________________________ and (10) ________________________. The more matter an object has, the larger its (11) _________________________. The more space an object takes up, the larger its (12)_________________________. Matter has both (13) _________________________ and (14) _________________________ properties. A (15) _________________________ property can be observed or measured without changing the identity of the object. Any change in a physical property is known as a (16) _________________________. In contrast, a (17) _________________________ is a change that produces a new substances. Another term for this type of change is a (18) _________________________. Matter: Fill in the information missing below State of Matter Solid Space between Atoms 21. Description of substance 23. 25. Label the parts of the atom. A. B. C. D. 19. 20. 22. A lot No definite shape, definite volume 24. Name _________________________________ Date_________________ Hour____________ Table #________ Qualitative or Quantitative? Write qualitative or quantitative next to each statement describing a candle burning. Be ready to discuss the reasons for your choices! 26. ____________________ The candle is cylindrical in shape. 27. ____________________ It is about 2 centimeters in diameter. 28. ____________________ The length decreased during the observation period. 29. ____________________ The amount of decrease was about .5 centimeters per half hour Above each graduated cylinder, label the total liquid volume shown in the cylinder. Use displacement volume to determine the volume of the irregular object. Below the cylinder record the volume of the object that has been placed in the second cylinder. No Naked numbers… remember your units! 33. Circle each of the following that IS an example of physical change. Put an X over those that are an example of a chemical change. Broken plates A Broken bottle B Crayons melting C Chains rusting E D Match burning Paper torn F Burning Candle G Boiling Water H Carved Pumpkin J I Cookies baking Name _________________________________ Date_________________ Hour____________ Table #________ 34. At which point is there the most potential energy? 35. At which point(s) is there kinetic energy? 36. At what point is energy being increased? 37. At what point is energy being decreased? 38. At what point is there the most potential energy? 39. At what point is there the a change from potential to kinetic energy? 40. At what point is gravity acting on the ball? 41. A flowering plant grew 10 centimeters in a two-week period and the color of its flowers changed from white to a pale yellow. Which of the following is true? A. These are qualitative changes. B. These are quantitative changes. C. The plant’s amount of growth is a quantitative change and the plant’s change in color is a qualitative change. D. The plant’s amount of growth is a qualitative change and the plant’s change in color is a quantitative change. Name _________________________________ Date_________________ Hour____________ Table #________ 42. Scientists collected this pollution data before, during, and after a volcanic eruption. Pollution data are measured in particles per liter. According to the graph, when did the eruption occur? A. between days 3 and 4 B. between days 5 and 6 C. between days 10 and 11 D. between days 14 and 15 43. Between points 2 and 3, energy is being used to change water from a A. B. C. D. Solid to a liquid Liquid to a solid Solid to a gas Liquid to a gas Solids Metals Liquids Study the Density Table Bone…….2 Aluminum…2.7 Pure water… 1 Brick….1.8 Copper…. 8.9 Sea water…1.03 44. Name two substances that will float on water. A. Cork… 0.2 Gold….19.3 Alcohol …. 0.8 Ice… 0.92 Iron…..7.8 Glycerin ….1.3 Marble….2.7 Lead … 11.3 Milk …. 1.03 Paraffin … 0.9 Silver ….10.5 Turpentine … 0.9 B. Rubber …1.2 Mercury …13.6 Bamboo… 0.3 Gasoline …. 0.7 Oak Wood …0.7 Pine Wood … 0.6 45. Name two substances that will sink in water. A. B.