GENERAL CHEMISTRY II CHEM 1312.005 EXAM 2 Thursday
advertisement

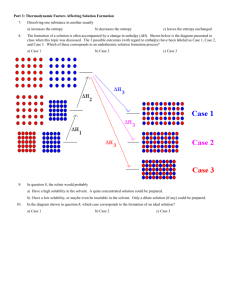
GENERAL CHEMISTRY II CHEM 1312.005 EXAM 2 Thursday, March 7, 2013 Name______________________________________ Banner ID__________________________________ 1. The illustration below was presented in class when discussing the thermodynamics of solution formation. For which of the cases shown in the diagram is the solute likely to have a low solubility (or even be insoluble) in the solute? a) Case 1 2. b) Case 2 c) Case 3 What situation is represented by “Case 1” in the above image? a) Endothermic solution formation b) Exothermic solution formation c) Solution formation that is neither endothermic nor exothermic 3. What do you call a solution in which all attractive forces (solvent-solvent, solute-solute, solvent-solute) are equal? a) An equitable solution b) An ideal solution d) A magic solution e) A pure solution c) A good solution 4. Dissolving one substance in another usually a) decreases the entropy 5. d) 5.93 M e) 7.67 M b) 2.95 m c) 4.02 m d) 5.21 m e) 6.98 m b) 1.73 m c) 2.44 m d) 3.55 m e) 4.87 m b) 0.248 c) 0.359 d) 0.451 e) 0.592 b) 0.221 c) 0.306 d) 0.445 e) 0.610 b) 16.4% c) 22.7% d) 34.4% e) 42.3% What is the molarity of potassium iodide (KI) in a 4.614 m aqueous potassium iodide solution? The density of this solution is 1.461 g/mL. Atomic weights: H 1.008 O 16.00 K 39.10 I 126.90 a) 1.215 M 13. c) 4.17 M What is the mass percent of glucose (C6H12O6) in a solution prepared by dissolving 41.2 g of glucose in 78.4 g of water? a) 11.5% 12. b) 2.79 M What is the mole fraction of ethanol (C2H5OH, or in more compact form, C2H6O) in a solution prepared by dissolving 24.5 g of ethanol in 75.2 g of water? Atomic weights: H 1.008 C 12.01 O 16.00 a) 0.113 11. e) 5.12 M What is the mole fraction of methanol (CH3OH, or in more compact form, CH4O) in a solution prepared by dissolving 0.419 moles of methanol in 0.511 moles of water? a) 0.117 10. d) 3.85 M An aqueous solution of NaI was prepared by dissolving 34.88 g of NaI in 95.5 g of water. What is the molality of NaI in this solution? Atomic weights: Na 22.99 I 126.90 a) 1.16 m 9. c) 2.71 M An aqueous solution of KBr was prepared by dissolving 0.335 mol KBr in 179 g of water. What is the molality of KBr in this solution? a) 1.87 m 8. b) 1.87 M An aqueous solution of CaCl2 was prepared by dissolving 11.52 g of CaCl2 in enough water to produce 37.2 mL of solution. What is the molarity of CaCl2 in this solution? Atomic weights: Ca 40.08 Cl 35.45 a) 1.53 M 7. c) leaves the entropy unchanged An aqueous solution of NaCl was prepared by dissolving 0.237 mol NaCl in enough water to produce 87.3 mL of solution. What is the molarity of NaCl in this solution? a) 1.09 M 6. b) increases the entropy b) 2.516 M c) 3.817 M d) 5.953 M e) 8.183 M What is the molality of potassium fluoride (KF) in a 9.832 M aqueous potassium fluoride solution? The density of this solution is 1.341 g/mL Atomic weights: H 1.008 O 16.00 F 19.00 K 39.10 a) 2.287 m b) 4.823 m c) 5.992 m d) 8.742 m e) 12.77 m 14. What is the mole fraction of glucose (C6H12O6) in a 2.973 m aqueous glucose solution? Atomic weights: H 1.008 C 12.01 O 16.00 a) 1.122 x 10-2 b) 2.773 x 10-2 c) 5.084 x 10-2 d) 7.629 x 10-2 e) 9.583 x 10-2 15. Consider a solution prepared by dissolving 50.00 g of glycerol, C3H8O3 (solute) in 50.00 g of water, H2O (solvent). At 50 oC, the vapor pressure of pure water is 92.5 torr. Assuming that glycerol is non-volatile and the solution obeys Raoult’s Law, what is the equilibrium vapor pressure of the solution at 50oC? Atomic weights: H 1.008 C 12.01 O 16.00 a) 31.5 torr 16. d) 77.4 torr e) 86.2 torr b) 99.7 g/mol c) 153 g/mol d) 195 g/mol e) 244 g/mol An aqueous solution of a protein was prepared by dissolving 1.75 g of a protein having a molecular weight of 27400 g/mol in enough water to produce 89.5 mL of solution. What is the osmotic pressure of this solution at 25 oC? R = 0.08206 L atm / K mol. a) 9.83 torr 18. c) 65.1 torr Pure cyclohexane has a normal freezing point of 6.55 oC. When a 0.387 g sample of a nonvolatile, non-ionizing solute was dissolved in 20.00 g of cyclohexane, the solution had a freezing point of 2.67 oC. The freezing point depression constant (Kf) for cyclohexane is 20.0 oC/m. What is the molecular weight of the solute? a) 64.5 g/mol 17. b) 55.3 torr b) 13.3 torr c) 19.2 torr d) 27.4 torr e) 36.1 torr A 2.08 g sample of a protein was dissolved in enough water to produce 97.3 mL of solution. The solution was found to have an osmotic pressure of 5.77 torr at 25 oC. What is the molecular weight of the protein? R = 0.08206 L atm / K mol a) 1.28 x 104 g/mol e) 3.03 x 105 g/mol b) 3.17 x 104 g/mol c) 6.89 x 104 g/mol d) 9.73 x 104 g/mol 19. The illustration shown below was presented in class when discussing Raoult’s Law. Which graph depicts a solution in which the strongest attractive forces are those between solute molecules and solvent molecules? a) Graph a 20. b) Graph b c) Graph c In the illustration above, which graph depicts the situation in which the solute would likely have the lowest solubility in the solvent? a) Graph a b) Graph b c) Graph c