probability enthalpy
advertisement

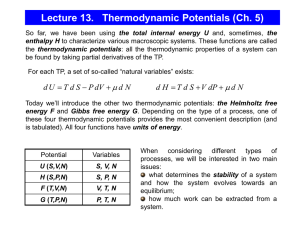
GROUP (8) CHAPTER FIVE AUXILIARY FUNCTIONS 2 5.1 Introduction The power of thermodynamics lies in its provision of criteria for equilibrium within a system and its ability to facilitate determination of the effect, on the equilibrium state, of change in the external influences which can be brought to bear on the system. The practical usefulness of this power is consequently determined by the practical of the equations of state of the system, i.e. the relationships among the state functions which can be established. The combination of the First and Second laws of Thermodynamics leads to the following equation: dU = TdS - pdV or U = f ( S, V ) This equation of state gives the relationship between the dependent variable U and the independent variable S and V for a closed system of fixed composition which is in states of equilibrium then undergoing a reversible process 3 involving volume change against external pressure as the only form of work performed on or by the system ( wp-v ) . The combination of the First and the Second Laws of Thermodynamics provides also the following criteria for the equilibrium : 1. for a closed system of constant energy and constant volume, the entropy is maximum, and 2. for a closed system of constant energy and constant volume, the internal energy is minimum. Since the entropy is an inconvenient choice as independent variable from the point of view of experimental measurements or control; it is desirable to develop a simple equation similar to the previous one which contains a more convenient choice of independent variables. From experimental point of view, the most convenient pair of independent variables would be temperature and pressure because they are the most easily measured and controlled parameters in a practical experiment. 4 From theoretical point of view, the most convenient pair of independent variables would be volume and temperature because when they are fixed for a closed system the ϵ(ί)’s level values ; and hence the Boltzmann factor ( exp ( ϵ(ί)’s / kT ) and the partition function are fixed; this will ease the theoritical calculations using statistical mechanics rules. Thus , in this chapter, to meet the previously discussed points the enthalpy function , H , the Helmholtz free energy function ( the work function ) , A , the Gibbs free energy function , G , and the chemical potential of species ί , μί , are introduced . 5.2 The Enthalpy H The enthalpy function H is defined as: H = U + PV thus: dH = dU + PdV + VdP = TdS – PdV + VdP + PdV 5 therefore: dH = TdS + VdP ί. e, H = ƒ ( S, P ) The dependent variable, in this case, is enthalpy while the pair of independent variables are the entropy and the pressure. Since the enthalpy is a state function: Δ H = H2 – H1 = ( U2 + P2V2 ) – ( U1 + P1V1 ) thus under constant pressure: Δ H = ( U2 – U1 ) + P ( V2 – V1 ) = Δ U - wp-v= qp Therefore, the equation of statue, dH = TdS + VdP , gives the relationship between the dependent variable H and the independent variables S and P for a closed system of fixed composition which is in state of equilibrium and is undergoing a reversible process involving volume change against external pressure as the only form of work performed on , or by , the system ; the enthalpy change of 6 the system equals the heat leaving or entering the system at constant pressure. 5.3 Helmholtz Free Energy Function A Helmholtz Free Energy Function A is defined as : A = U – TS thus: dA = dU – TdS – SdT = TdS – PdV – TdS – Sdt therefore dA = – SdT – PdV =ƒ(V, T) The dependent variable in this case is A and the pair of independent variables are volume and temperature. Since the Helmholtz free energy function is a state function, thus: Δ H = A2 – A1 = ( U2 – T2S2 ) – ( U1 – T1S1 ) 7 = ( U2 – U1 ) – ( T2S2 – T1S1 ) ί . e, Δ A = q + w – ( T2S2 – T1S1 ) thus: Δ A – w = q – ( T2S2 – T1S1 ) In describing the work in the previous equation, a positive sign is assigned to work done on the system and a negative sign is assigned to work done by the system. thus: - w – ( - ΔA )= q – ( T2S2 – T1S1 ) If the process is isothermal; that is T2 = T1 = T, then: -w – ( - Δ A ) = q – T ( S2 – S1 ) From the second law of thermodynamics : q ≤ T ( S2 – S1 ), thus - w ≤ –Δ A Therefore, for reversible isothermal process : wmax = – Δ A ί.e. the maximum amount of work done by system equal the decrease in the work function . since: w =wmax–w deg then: w = –Δ A–TΔSirr 8 = – ( ΔA + T ΔSirr ) Thus, for a process which occurs at constant V and T, we can write: Δ A + T Δ Sirr = 0 for an infinitesimal increment of such process: δA + TdSirr = 0 We know that for spontaneous processes dSirr is a positive value , thus processes occur at constant T and V will be spontaneous if dA is a negative value ; ί.e, for spontaneous processes that occur at constant T and V : dA<0 Since the condition for thermodynamic equilibrium is that dSirr = 0 then with respect to the described process. ί. e , at constant V and T equilibrium is defended by the condition dA<0 Thus, for a closed system , held at constant T and V, Helmholtz free energy can only decrease ; for a spontaneous process, the attainment of equilibrium in the 9 system coincides with the system having a minimum value of A is corresponding to the fixed values of V and T Consideration of A thus provides a criterion of equilibrium for a closed system with fixed composition at constant value V and T. Consider for example n atoms of some element occruing in a crystalline phase and a vapor phase both contained in a constant – volume vessel which is immersed in constant – temperature heat reservoir. The point now is to determine the equilibrium distribution of the n atoms between the sold phase and the vapor phase. At constant V and T, this distribution occurs at the minimum value of A, and hence with low value of U and high value of S since: A=U - TS The two extreme states of existence of this system are : 1. All n atoms are in the solid phase and none occur in the vapor Phase. 10 2. All n atoms are in the vapor phase and the solid phase is absent. Starting with the system occurring in the first of these two states, ί. e, the solid crystalline state ; the atom in such case are held together by interatomic force ; thus , if an atom to be removed from the crystal surface and placed in the vapor phase ( the first atom is placed in vacuum ) , energy is absorbed as heat from the heat reservoir to the system to break the interatomic bonds to increase the internal energy , U , of the system, and its randomness ί.e, the system entropy as shown in figure (5.1 ) which shows the variation of internal energy and entropy with the number of atoms in the vapor phase of the closed solid vapor system at constant V and T . 11 u s nv Figure(5.1): the dependence of U and S on nv. 12 This figure shows that U increases linearly with nv while the entropy increase is nonlinear. The saturated vapor pressure is calculated as : P = [ nv ( eq , T ) kT ] / [ v-vs ] Where V is the volume of the containing vessel, Vs is the volume of the solid phase present, and nv ( eq , T ) is the number of atoms in the vapor phase at the equilibrium point which correspond to the minimum value of A as shown in figure (5.2 ); this minimum value is obtained by adding the values of U to the corresponding values of ( – TS ) and thus having a curve that represents the variation of A with nv. 13 U A=U-TS -TS Figure(5.2): the variation of U, TS and A. 14 As the magnitude of the entropy contribution to the value of A, –TS, is temperature dependent and the internal energy contribution is independent of temperature, the entropy contribution becomes increasingly predominant as temperature is increased and the compromise between U and ( – TS ) which minimize A occurs at higher value of nv. This is illustrated in (5.3 ) which is drawn for T1 and T2 where T2 > T1 15 U _________________________________________ A=U-T1s A=U-T2s -T1s -T2s Figure(5.3): the variation of A with nv. at T1 and T2 where T2>T1 . 16 The increase in temperature from T1 to T2 increases the saturated vapor pressure: from to p( T1 ) = nv ( eq,T1 ) kT1 / [ V–Vs( T1 ) ] p( T2 )= nv ( eq,T2 ) k T2 [V–Vs(T2)] The saturated vapor pressure increases exponentially with increasing the temperature. For the constant – volume system, the maximum temperature at which both solid and vapor phases are in equilibrium occurs at nv ( eq,T ) = n; above this temperature, the entropy contribution overwhelms the internal energy contribution and hence all n atoms occur in the gas phase. Conversely, as T decreases, then nv ( eq,T ) decreases and, in the limit that T = 0K, the entropy contribution to A vanishes and minimization of A coincides with minimization of U , that is all n atoms occur in the solid phase . Now consider that the constant temperature heat reservoir containing the constant volume system, is of constant volume and is adiabatically contained, then the combined 17 system, the particles containing system and the heat reservoir, is one of constant U and V; accordingly the combined system attains equilibrium at its maximum point of entropy. If nv < nv ( eq ,T ) , the evaporating process occurs , this process is accompanied by transfer of heat q , from the heat reservoir to the particles containing system , thus the entropy change of the combined system is given by : Δ S combined system = ΔS heat reservoir +ΔS particles system = – q/T + ( q/T + ΔSirr ) = ΔSirr thus: Δ A = – TΔSirr Since minimization of A correspond to maximization of entropy Also if nv > nv ( eq,T ) condensation will occur ; the entropy change of the combined system , in this case , is given by : Δ S combined system = ΔS heat reservoir + ΔS particles system = +q/T + ( - q/T + ΔSirr ) 18 = ΔSirr thus: Δ A = – TΔSirr Since at equilibrium: thus: ΔSirr = 0 ΔA = 0 It should be noted that at, or near, equilibrium the probability that nv deviates by even the smallest amount from the value nv ( eq,T ) exceedingly small. This probability is small enough that the practical terms it corresponds to is the thermodynamic statement “spontaneous deviation of a system from its equilibrium state is impossible". 19