Hemorrhage Outcome Measures For HENs - K-HEN
advertisement

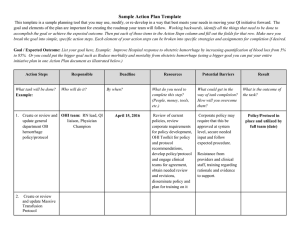
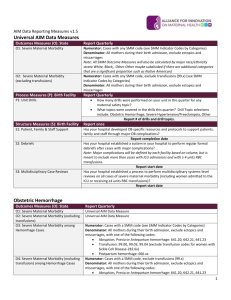
OB Hemorrhage OUTCOME Measures For Multi-hospital QI Projects CMQCC Recommendations: Dec 19, 2013 The focus of obstetric hemorrhage QI collaborations to date has been to reduce maternal morbidity by early identification and a standardized and timely response. Morbidity has measured by the number of blood units used and the frequency of peripartum hysterectomies Timely response can often include early rather than late transfusions in order to avoid disseminated intravascular coagulation (DIC). The two measures, total number of blood products used and the number of mothers who require large numbers (≥4) of transfusions, have been validated by several large multi-hospital obstetric hemorrhage QI collaboratives. Their results indicate that reduction by 20-30% is achievable. These studies also did not find any evidence of an unintended consequence of providers avoiding a needed transfusion to meet the quality guideline. Importantly, the new Joint Commission definition of an Obstetric Sentinel event will include transfusion of ≥4 units of blood products. This will reinforce the importance and the collection of this measure. This target is based on the observation that real patient harm is observed with higher number of transfusions and not with 1 or 2 units. Regarding the measure of the number of women receiving any transfusions (including 1 or 2 units), we are unaware of any hemorrhage QI study that was able to identify a reduction in this metric. Furthermore, we have real concerns for unintended consequences (with potential harm) as such a goal could lead to pressures on providers not to provide or to delay a needed first unit of blood. An additional consideration is that in recent years, physicians have been strongly encouraged to refer women with major placental complications such as placenta accreta to regional centers. These patients usually require multiple units of blood products and often a hysterectomy. Multiple studies have identified that with a multi-disciplinary treatment plan the number of units of blood products can be significantly reduced, sometimes even below the 4-unit threshold. This supports the focus on the number of units used rather than the number of patients transfused. It also highlights the need to look at blood products regionally as often tertiary centers will have higher utilization based on case mix. Other outcome measures can be considered but are either uncommon (peripartum hysterectomy) or not yet validated (Callaghan/CDC measure of severe maternal morbidity). It is also anticipated that many collaboratives may also want to locally use process measures and “deliverable lists” to support the change process, these are not discussed here. Note these measures are for collaboratives and similar QI projects (such as Hospital Engagement Networks) that utilize time course designs. They are not designed or validated as inter-hospital quality measures. Measure specifications: 1. Total number of transfusions Short Description: Total number of blood products transfused per 1,000 mothers Denominator: All women giving birth ≥20 weeks (birth hospitalization) Numerator: Total number of units of blood products (RBCs, FFP, Plt packs, Cryo) Expected Baseline Rate: 40-60 units per 1,000 mothers Source: Hospital Blood bank data sets or ChargeMaster 1 2. Number of massive transfusions Short Description: Number of mothers receiving 4 or more units of blood products per 1,000 mothers Denominator: All women giving birth ≥20 weeks (birth hospitalization) Numerator: Women who received ≥4 units of blood products (including RBCs, FFP, Platelet packs, Cryoprecipitate) (This is also the new definition of an Obstetric Sentinel Event by The Joint Commission so it will be required to be captured by TJC accredited hospitals) Expected Baseline Rate: 2-4 cases per 1,000 mothers (may be higher) Source: Hospital Blood bank data sets or ChargeMasters Comments: (1) the denominator is restricted to ≥20 weeks of gestation as that defines the typical labor and delivery population. While earlier pregnancies do have hemorrhages (e.g. ectopic and late miscarriages), these are quite uncommon and typically have different etiologies and would require a different QI project with a focus on different care venues (office, ER, OR). Furthermore, there is no good way of properly identifying the denominator population for earlier gestations— all pregnancies? or all pregnancies beyond 8 weeks? Etc. (2) The numerator identifies all blood products rather than just RBCs. This is the definition used by the Joint Commission and supported by an ACOG/CDC/SMFM consensus committee (in press). (3) Units of blood products is a reasonable measure to collect using Blood Bank databases or Charge Masters. After discussion with an analyst, most hospitals can create a monthly report of patients transfused with the number of units per patient. (4) The Joint Commission declaration of ≥4 units will be a powerful tool to help drive this quality initiative and it will be important to have coordinated outcome measures. REFERENCES: Callaghan WM, Mackay AP, Berg CJ. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991-2003.Am J Obstet Gynecol. 2008 Aug;199(2):133.e1-8. Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012 Nov;120(5):1029-36 Lagrew D, Lyndon A, Melsop K, Main EK. Results from first 3 CMQCC Hemorrhage Collaboratives. Manuscript in preparation (2013) Shields LE, Smalarz K, Reffigee L, Mugg S, Burdumy TJ, Propst M. Comprehensive maternal hemorrhage protocols improve patient safety and reduce utilization of blood products. Am J Obstet Gynecol. 2011 Oct;205(4):368.e1-8. Shields LE, Chagolla B, Fulton J, Pelletreau B. Comprehensive maternal hemorrhage protocols reduce utilization of blood products and improve patient safety. Am J Obstet Gynecol 2013 Jan;208(1 supplement):S49-50. 2