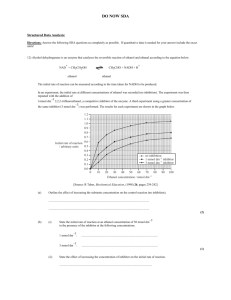
Electronic supplementary material Synthesis of the esters 7
advertisement

Electronic supplementary material Synthesis of the esters 7-methyloctyl 5-methylhexanoate, 7-methyloctyl octanoate, 7-methyloctyl 7-methyloctanoate, and 7-methyloctyl (Z)-4-decenoate: General procedure: The esters were synthesized using a modified method of that described by Tolasch et al. (2007). Commercially available chemicals were used without further purification. 5-Methylhexanoic acid, 5-methylhexyl bromide and (Z)-4-decen-1-ol were purchased from Alfa Aesar. Tetrahydrofuran (THF), dimethyl sulfoxide (DMSO), dichloromethane (CH2Cl2) and pyridine was dried and/or distilled prior to use. Preparative liquid chromatography was performed on normal-phase silica gel (Merck 60, 230-400 mesh, 0.040-0.063 mm, 10-50 g/g of product mixture) employing a gradient technique with an increasing concentration (0-100%) of distilled diethyl ether in distilled pentane. Thin-layer chromatography (TLC) was performed to monitor the progress of the reactions on silica gel plates (Merck 60, pre-coated aluminium foil), using ethyl acetate (40%) in cyclohexane as an eluent, and plates were visualised by means of ultraviolet irradiation and/or by spraying with vanillin in sulfuric acid and heating at 120°C. Purity of the product was checked with GC analysis on a Varian 3300 GC instrument (Varian, Palo Alto, CA, USA) using an EC-1 column (30 m x 0.32 mm ID, 0.25 μm film thickness (Alltech, Deerfield, IL, USA)). Nitrogen was used as carrier gas. The column temperature was maintained at 50°C for 5 min and then increased by 10°C/min to 300°C. Mass spectral analyses were carried out on a Saturn 2000 GC/MS/MS instrument (Varian), coupled to a Varian 3800 GC instrument (Varian), using a CP-Sil 5 CB column (30 m x 0.32 mm ID, 0.25 μm film thickness (Varian)). Helium was used as carrier gas at a flow rate of 1 ml/min. The same temperature program was used as above. NMR spectra were recorded on a Bruker Avance 500 (500 MHz 1H, 125.8 MHz 13C) spectrometer (Bruker, Solna, Sweden) using CDCl3 as solvent and TMS as internal standard. 7-Methyloctan-1-ol NaH (2.7 g, 111 mmol) was added in portions to a solution of dimethyl malonate (14.7 g, 111 mmol) and THF (250 mL) at 0°C. The mixture was stirred for 10 min before drop-wise adding 5methylhexyl bromide (2.92 g, 16.3 mmol) in THF (50 mL). The reaction was set on reflux overnight, and then quenched with NH4Cl (110 mL) and extracted with EtOAc (3 x 50 mL). The combined organic layers were dried (MgSO4) and evaporated. Most of the excess of dimethyl malonate was removed by distillation under reduced pressure, using a vigreux column. The crude malonic ester was then purified by flash chromatography and checked by NMR. The crude malonic ester (3.3 g, 14.3 mmol) in DMSO (6 mL) was added to a mixture of NaCl (0.84 g) and H2O (1.0 mL) in DMSO (12 mL) and refluxed over night. The reaction was quenched with H2O and extracted with EtOAc (2 x 25 mL). The combined organic layers were dried (MgSO4), concentrated and after flash chromatography a pale yellow oil (2.0 g, 11.6 mmol) was obtained. The obtained methyl ester was checked by NMR and used in the next step without further purification. The product ester (1.7 g, 9.9 mmol) in THF (40 mL) was added drop-wise to a stirred mixture of THF (40 mL) and LiAlH4 (0.76 g, 9.9 mmol) at 0°C. The reaction was allowed to reach room temperature overnight. The reaction was quenched with NH4Cl and HCl (2 M) at 0°C and extracted with EtOAc (3 x 50 mL). The combined organic layers were washed with brine (20 mL), dried (MgSO4) and the solvent evaporated. The pale oil was purified by bulb-tobulb distillation (130°C, 2 mbar) to yield the pure (GC) product alcohol (1.29 g, 8.96 mmol) as a colourless oil in 55% from the bromide. The analytical data were accordingly to those reported in the literature (Tolasch et al. 2007). (Z)-4-decenoic acid (Z)-4-decen-1-ol (3 g, 19.2 mmol) in acetone (160 mL) was stirred on an ice bath and Jones reagent (28.8 mmol) was added at a temperature below 5°C. After 1 h the reaction was quenched by adding water (8 mL). The reaction was extracted with Et2O (3 x 50 mL) and the combined organic layers were washed with a water solution of Na2CO3 (3 x 30 mL), dried (Na2SO4) and the solvent evaporated. The residue was purified by bulb-to-bulb distillation to yield the product acid as an almost colourless oil (17 mmol, 89%). The analytical data were accordingly to those reported in the literature (Tolasch et al. 2007). 7-metyloctanoic acid The product acid was synthesized as above for (Z)-4-decenoic acid, but starting from 7methyloctan-1-ol. The analytical data were accordingly to those reported in the literature (Tolasch et al. 2007). 7-Methyloctyl (Z)-4-decenoate (Z)-4-decenoic acid (0.46 g, 2.71 mmol) in SOCl2 (2.2g, 1.4 mL, 18.9 mmol) was refluxed for 1 h. The excess SOCl2 was then removed by distillation and the obtained acid chloride was then distilled bulb-to-bulb and immediately used in the next step. The acid chloride (0.51 g, 2.71 mmol) in CH2Cl2 (4 mL) was added drop-wise to a stirred solution of 7-methyloctan-1-ol (0.50 g, 2.7 mmol) in CH2Cl2 (4 mL) and pyridine (0.255 g, 3.0 mmol) under argon atmosphere. The reaction was stirred overnight, during which it becomes slightly cloudy, and was then quenched with water. The reaction was extracted with Et2O (3 x 20 mL) and the combined organic layers were dried (MgSO4) and the solvent evaporated. The residue was purified by flash chromatography to yield the product ester pure (GC) as a colourless oil (2.38 mmol, 88%). The analytical data were accordingly to those reported in the literature (Tolasch et al. 2007). 7-Methyloctyl octanoate The product ester was synthesized as above for 7-methyloctyl (Z)-4-decenoate but starting from 7-methyloctan-1-ol and octanoic acid. The analytical data were accordingly to those reported in the literature (Tolasch et al. 2007). 7-Methyloctyl 7-methyloctanoate The product ester was synthesized as above for 7-methyloctyl (Z)-4-decenoate, but starting from 7-methyloctan-1-ol and 7-metyloctanoic acid. The analytical data were accordingly to those reported in the literature (Tolasch et al. 2007). 7-Methyloctyl 5-methylhexanoate The product ester was synthesized as above for 7-methyloctyl (Z)-4-decenoate, but starting from 7-methyloctan-1-ol and 5-methylhexanoic acid. The analytical data were accordingly to those reported in the literature (Tolasch et al. 2007).