Supplementary Materials for: Synthetic Molecular Gear based on
advertisement

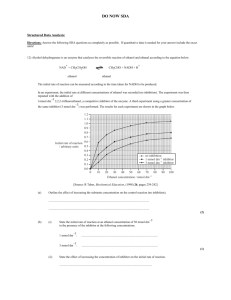
Supplementary Materials for: Synthetic Molecular Gear based on Double-decker Porphyrin Complexes Soichiro Ogi, Tomohiro Ikeda, Masayuki Takeuchi Organic Materials Group, Polymer Materials Unit, National Institute for Materials Science (NIMS) 1-2-1 Sengen, Tsukuba, Ibaraki 305-0047, Japan TAKEUCHI.Masayuki@nims.go.jp –S1– This SI describes the synthetic procedures and characterization data for the precursors of compound DD1, 1, 2. Compound S2 and S3 were synthesized according to our previously reported procedure.1 All other reagents and solvents were purchased from commercial sources and used without further purification. Air- and/or water-sensitive reactions were conducted under argon using dry solvents. Scheme S1. –S2– 5-(4-pyridyl)-15-(4-methoxyphenyl)-21H,23H-porphine (S1): A solution of 2,2’-dipyrromethane (2.14 mg, 14.6 mmol), p-anisaldehyde (0.89 mL, 7.3 mmol), and 4-pyridinecarboxaldehyde (0.70 mL, 7.3 mmol) in CH2Cl2 (1.83 L) was bubbled with argon for 1 hours. Trifluoroacetic acid (2.70 mL, 35.1 mmol) was added to the solution under argon atmosphere. After stirring for 21.5 hours at room temperature, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (6.63 g, 29.2 mmol) was added and the mixture was stirred for another 30 minutes. The reaction mixture was neutralized with trimethylamine (6.00 mL) and washed with water, and then the organic layer was dried over anhydrous Na2SO4. The filtrate was evaporated in vacuo and obtained material was purified by column chromatography (SiO2; CH2Cl2/MeOH, from 1:0 to 50:1) and reprecipitation procedure (MeOH) to yield compound S1 as a purple-red powder (127 mg, 3.5%). H NMR (CDCl3, 298 K): δ –3.13 (2H, s, inner-NH), 4.14 (3H, s, Ar–OMe), 7.37 (2H, d, J = 8.7 Hz, l o-H in C6H4–OMe), 8.20 (2H, d, J = 8.4 Hz, m-H in C6H4–OMe), 8.23 (2H, d, J = 5.0 Hz, o-H in C5H4N), 9.04 (2H, d, J = 4.5 Hz, β-pyrrole), 9.08 (2H, d, J = 5.7 Hz, m-H in C5H4N), 9.14 (2H, d, J = 4.5 Hz, β-pyrrole), 9.42 (2H, d, J = 4.5 Hz, β-pyrrole), 9.44 (2H, d, J = 4.8 Hz, β-pyrrole), 10.4 (2H, s, meso-H). MALDI-TOF-MS (Matrix: dithranol): Found m/z = 493.61, Calcd for [M]+ (C32H23N5O) = 493.19. 3,5-bis(trimethylsilylethynyl)benzaldehyde (S4): A mixture of 3,5-dibromobenzaldehyde (818 mg, 3.10 mmol), dichlorobis(triphenylphosphine)palladium(II) (313 mg, 0.45 mmol), and copper iodide (44 mg, 0.23 mmol) in diisopropylamine (12 mL) and THF (24 mL) was stirred at 50 °C for an hour under argon atmosphere. Trimethylsilylacetylene (913 mg, 9.3 mmol) was added to the solution with stirring and the resultant mixture was stirred at 80 °C for 10 hours. After cooling to room temperature, the reaction was diluted with n-hexane and washed with water, and then the organic layer was dried over anhydrous Na2SO4. The filtrate was evaporated in vacuo and obtained liquid material was purified by column chromatography (SiO2; n-hexane/ethyl acetate, 9:1) to yield compound S4 as a –S3– light-yellow oily matter (458 mg, 49%). H NMR (CDCl3, 298 K): δ 0.25 (18H, s, SiMe3), 7.79 (1H, t, J = 1.8 Hz, p-H in C6H3–CHO), 7.88 l (2H, d, J = 1.8 Hz, o-H in C6H3–CHO), 9.94 (1H, s, Ar–CHO). 5,10,15,20-tetrakis[3,5-bis(trimethylsilylethynyl)phenyl]-21H,23H-porphine (S5). A solution of S4 (668 mg, 2.23 mmol) in propionic acid (8 mL) was stirred at 80 °C for an hour. Pyrrole (0.15 mL, 2.23 mmol) was added and the mixture was stirred under reflux for 3 hours while shielded from the light. After cooling to room temperature, the reaction solution was purified through reprecipitation method (MeOH) and column chromatography (SiO2; n-hexane/CH2Cl2, 3:7) to yield compound S5 as a purple powder (114 mg, 15%). H NMR (CDCl3, 298 K): δ –2.95 (2H, s, inner-NH), 0.26 (72H, s, SiMe3), 8.03 [4H, t, J = 1.2 Hz, l p-H in C6H3(C≡C–SiMe3)2], 8.23 [8H, d, J = 1.2 Hz, o-H in C6H3(C≡C–SiMe3)2], 8.82 (8H, s, β-pyrrole). MALDI-TOF-MS (Matrix: dithranol): Found m/z = 1382.38, Calcd for [M]+ (C84H94N4Si8) = 1382.56. 5,10,15,20-tetrakis[3,5-bis(trimethylsilylethynyl)phenyl]-21H,23H-porphyrinato rohodium(III) chloride (S6): A solution of compound S5 (57.0 mg, 41.2 μmol) and tetracarbonyldi-μ-chlorodirhodium(I) (33.8 mg, 86.9 μmol) in dry toluene (100 ml) was stirred at 80 °C for 8 hours under argon atmosphere. After the solvent was evaporated, the residue was purified through column chromatography (SiO2; n-hexane/CH2Cl2, from 7:3 to 1:1) to yield compound S6 as a red powder (6.9 mg, 11%). MALDI-TOF-MS (Matrix: dithranol): Found m/z = 1483.72, Calcd for [M–Cl]+ (C84H92N4RhSi8) = 1483.45. –S4– Fig. S1. 1H NMR spectrum of 1 in chloroform-d1 at 253 K. –S5– Fig. S2: 1H-1H 2D COSY spectrum of 1 in chloroform-d1 at 253 K. -----------------------------------------------------------------------------------------------------------------------Reference 1. S. Ogi, T. Ikeda, R. Wakabayashi, S. Shinkai, M. Takeuchi, Chem. Eur. J. 16, 8285 (2010) –S6–