Here
advertisement

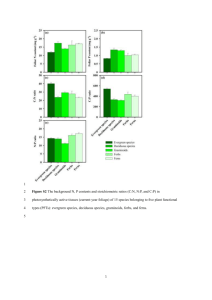
Role of plankton functional diversity for marine ecosystem services SUGGESTION OF ALTERNATIVE TITLE: Functional diversity of marine plankton: effects on ecosystem services in the global ocean Corinne Le Quéré, Erik T. Buitenhuis, Rósín Moriarty, Meike Vogt, Sévrine Sailley, Sophie Chollet, Nick Stephens, Clare Enright, Dan Franklin, Stuart Larsen, Louis Legendre, Trevor Platt, Richard B. Rivkin, Shubha Sathyendranath Other invited authors to be confirmed: Severine Alvain, Olivier Aumont, Laurent Bopp, Richard Geider, Sandy P. Harrison, Christine Klaas, I. Colin Prentice, Andy J. Watson, Dieter Wolf-Gladrow To be submitted either to either Science as a Review (3500 words incl references) or Research Article (up to 4500) or to Global Change Biology (up to 6500 words). Current word count: 4375+references. Abstract (<200 words version) Much work has been done to understand and quantify the effects of plant functional diversity on ecosystem services on land. In the ocean however, quantitative evidence of such effects is almost entirely lacking. Here we present a synthesis of vital rates for ten plankton functional groups that play key roles for marine ecosystem services at the global scale. We use these rates to build a global ecosystem model, and independent biomass observations to validate it. We use the model to quantify the effects of different plankton groups on primary, secondary, and export production – which are crucial for such ocean services as food production and climate feedbacks. Increasing the diversity of modelled plankton improves the representation of observed biomasses and corrects important biases of simpler models. Model results suggest that primary production is more sensitive than secondary and export production to ecosystem perturbations because of the dampening effect of internal ecosystem feedbacks. Export production responds non-linearly to changes in plankton groups, indicating the presence of strong amplifying and dampening feedbacks in ecosystem dynamics. Identifying these feedbacks and the current state of marine ecosystems is essential to assess the limits of climate change that must not be exceeded. Introduction There is ample evidence from ocean observations that the diversity of marine plankton has large effects on marine biogeochemical cycles (ref) and ecosystem services [1]. Yet the specific role of plankton groups for ecosystem services has never been quantified because of the lack of systematic analysis of available data and model results. Marine plankton control important ecosystem services that include food production (provisioning service) and climate feedbacks (regulating service), which are supported by primary, secondary and export production in oceans, which are called supporting services. The production of organic matter by plants using CO2, water and inorganic nutrients, called primary production, serves as food for small organisms including bacteria, zooplankton and some fish larvae. The organic matter produced by zooplankton grazing on phytoplankton, called secondary production, serves directly or indirectly as food for other marine organisms, including fish and mammals. The fraction of primary production that sinks from surface toward depth, called export production, exerts a strong influence on marine biogeochemical cycles and climate because part of the CO2 resulting from the remineralisation of sinking organic matter is sequestered at depth where it becomes isolated from the atmosphere. Export production mainly reflects the activity of large phyto- and zooplankton. It lowers the surface concentration of carbon, and maintains atmospheric CO2 about 200 ppm lower than it would be in the absence of biological activity [2]. The organic matter recycled by living organisms in the surface ocean cannot be exported. Many phyto- and zooplankton taxa can be grouped into Plankton Functional Types (PFTs) according to their specific effects on marine biogeochemical cycles [3, 4]. Some PFTs have been represented in global biogeochemical models in the past. For example, the representation of diatoms helped to reproduce the observed response to iron fertilisation in the ocean [5], and changes in export production during glacial cycles [6]. Other models represented coccolithophores and cyanobacteria [7, 8] and different classes of zooplankton [9] with a mixture different growth rates, nutrient limitations or food preferences. Here we present a synthesis of rates of growth and other parameters for the 10 PFTs defined in [3]: picophytoplankton, N2-fixers, coccolithophores, mixed-phytoplankton, Phaeocystis, diatoms, bacteria, protozooplankton, mesozooplankton, and macrozooplankton. We used these rates as a basis to build a new global ocean biogeochemistry model, which we perturbed to identify the specific role of PFTs for ecosystem services. Materials and methods Source and treatment of observations The most important trait that distinguishes the various PFTs is the rate at which they grow under different conditions [9, 10]. We compiled specific growth rate values as a function of temperature for the ten PFTs. The data were either taken from previous compilations (see references in Table 1) or assembled here from literature review. The zooplankton data reported as grazing rates were converted to growth rate using a gross growth efficiency of 0.3 [9, 10]. The full list of references and the growth data are provided in the Supplementary material. The number of observations varied from 36 data points only for N2-fixers to 2745 data points for mesozooplankton (Figure 1; Table 1). The growth rates were as explained in Note 1. The data shows systematic patterns among PFTs, i.e. the growth rate increased with size for phytoplankton PFTs from 0.15 d-1 at 20°C for N2-fixers to 1.85 d-1 for Phaeocystis, and decreased with size for heterotrophic PFTs from 1.22 d-1 for bacteria to 0.19 d-1 for macrozooplankton (Table 1). This relationship with size is contrary to what has been observed amongst diatoms [11], suggesting that the specific set of ecological characteristics that distinguish PFTs (e.g. growth rates, nutrient requirements, zooplankton food preference) play a more important role for growth rate than cell thermodynamic properties (e.g. diffusion rates across cell walls). Phytoplankton growth is also limited by the availability of nutrients, and their resulting biomass is controlled by top-down pressure resulting from zooplankton. There are far less data available to constrain the growth dependence of PFT as a function of nutrient or food availability. To gain insight on the importance of limiting factors for growth, we examined the co-variance between phytoplankton biomass as captured indirectly by remotely sensed chlorophyll a (chla) and either nutrients or heterotrophic PFTs (Figure 2). The available data shows that chla co-varies with NO3- at concentrations below about 3 μmol/L, and with PO43- at concentrations between about 0.3 and 0.5 μmol/L. There is no covariance between chla and Fe concentration up to 1.0 nmol/L, contrary to what would be expected if Fe limitation had a dominant influence on phytoplankton biomass. The strongest covariance is found between chla and protozooplankton biomass, with chla varying between 0 and 0.7 mgChl/m3 for protozooplankton from 0 to 0.6 μmol/L. Covariance was also observed with bacteria and mesozooplankton, but not with macrozooplankton. The combined set of observations suggests an important role of nutrient regeneration by heterotrophic plankton in controlling surface chla concentration. Model description To understand the functional relationship between biogeochemical cycles and ecosystem diversity, we build a Dynamic Green Ocean Model representing the above 10 PFTs. The growth rate parameters for the “PlankTOM10” model were based on our compilation of growth rates as a function of temperature (Figure 1). Because limitation parameters are not well constrained by observations, we used the following two-tier approach. Firstly, we assigned limitation parameters to each phytoplankton PFT based on literature values. We assigned PFT-specific parameter values when published studies supported distinct values compared to other PFTs. In the absence of PFT-specific information, we assigned the same value as that of the similar-sized PFT. Secondly, we examined the co-variance of surface chla with each available limiting nutrients and heterotrophic PFTs and adjusted the amplitude of the limitation parameters of both phytoplankton and zooplankton PFTs with trial and error, keeping the ratios between PFTs approximately constant1. To better understand the role of plankton diversity, we constructed a simplified ‘PlankTOM6’ version of the model by lowering PFT diversity to include only six PFTs (diatoms, picophytoplankton, coccolithophores, bacteria, protozooplankton and mesozooplankton), i.e. removing four phytoplankton PFTs and macrozooplankton). Reduced PFT diversity was achieved by setting the growth rates of the other PFTs to zero, increasing the mortality of the top predator (mesozooplankton) until primary production was optimal (from 0.13 to 0.26 d-1), and setting the grazing preferences for the missing PFTs to zero. This model versions were identical in all other respects to PlankTOM10 and is similar in structure to the model of [10], but with explicitly bacterial biomass and activity and modified parameters. Results PlankTOM10 reproduces the main characteristics of observed surface chla, with high concentrations in the high latitudes and low concentrations in the tropics (Figure 3), which is at least as good as other simpler models [13]. The chla distribution is improved in PlankTOM10 compared to PlankTOM6, with sharper contrast between the very low chla concentrations in the sub-tropical gyres and the very high chla concentrations along the coasts and at high Northern latitudes, and with higher chla concentration in the Northern latitudes compared to the Southern Ocean. Correlations between PlankTOM10 and SeaWiFS data are r=0.56 (n=52591) and r=0.59 (n=730487) for the annual and monthly data, respectively, compared to r=0.44 and r=0.51 in PlankTOM6, respectively. The global biogeochemical fluxes are also in the bulk part of the observations, with global primary production at 38.2 PgC yr-1, export production at 7.3 PgC yr-1, and export of CaCO3 and SiO2 at 0.7 PgC yr-1 and 82 Tmol Si yr-1, respectively, although these fluxes are generally on the low end of the observations (Table S3). PlankTOM10 produces a distinctive distribution for all PFTs, with most of the phytoplankton biomass in picophytoplankton, and most of the zooplankton biomass in mesozooplankton (Figure 4). The phytoplankton biomass distribution is similar to that inferred from observations, but the zooplankton biomass distribution underestimates protozooplankton and overestimates mesozooplankton compared to available observations (Table S3). This problem could be caused by the presence of a range of grazers in the protozooplankton group, including nanozooplankton and protozooplankton with important feeding differences, by missing ecological characteristics for mesozooplankton including overwintering and vertical dial migrations, or by incorrect feeding preferences of some groups. In PlankTOM10 results, small phytoplankton are generally present in tropics, haptophytes (coccolithophores and Phaeocystis ) in mid latitudes, and diatoms in high latitudes. These patterns generally match the distribution of phytoplankton based on satellite normalised radiance (Figure S1), with the exception of the tropical Atlantic and Indian Oceans where the model produces a dominance of haptophytes because of the predominance of PO43- limitation, whereas estimates based on satellite data suggest a dominance of small phytoplankton. PlankTOM10 also reproduces the general distribution of blooms of Phaeocystis and coccolithophores (Figure S2), again with an overestimation of coccolithophores in the tropical Atlantic. The fit of model results to available data is summarised in a Taylor diagramme (Figure S3). As expected because of the initialisation procedure, the model fits best the macronutrient fields. The model shows skill in reproducing the amplitude and correlation of chla, primary production and export production, and several other biomass components. The model skills are very low (r<0.2) for Fe and the biomass distribution of proto- and macrozooplankton, which are more patchy in nature than the nutrients and phytoplankton. Overall, the model reproduces well the general patterns and amplitudes of the biomass, fluxes, and covariance between variables (Figure S4), but it does not match all details of the observations. In particular, the model has a bias in the tropical representation of phytoplankton groups in the Atlantic ocean, overestimates the biomass of mesozooplankton and underestimates protozooplankton, and underestimates the co-variance of chla with mesozooplankton. The study includes sensitivity tests, which are performed to assess the effets of model deficiencies on the results and are discussed below. Discussion Importance of PFT diversity for model representation of surface chlorophyll a The presence of 10 PFTs in PlankTOM10 improves the representation of the mean and seasonality of chla relative to models with less PFTs, and corrects important biases in the representation of chla. The improvements in the representation of the low chla concentration appear related to the diversity of the phytoplankton PFTs, i.e. the lowest chla reproduced by the model is 0.05 and 0.01 mgChl m-3 for PlankTOM6 and PlankTOM10, respectively (Figure S5). Additional experiments where we added macrozooplankton to PlankTOM6 or removed macrozooplankton from PlankTOM10 did not modify the minimum modelled chla concentration (not shown). The wider range of chla produced by the more diverse model is closer in amplitude and in structure to the observations. The model results suggest that increased PFT diversity enhances the capacity of the ecosystem to better exploit the range of ecological niches present in the ocean. The higher ecosystem diversity also corrects previous biases in the chla balance between the two hemispheres. Observations show higher chla concentration in the Northern Hemisphere compared to the Southern Hemisphere (Figure 3), which has been attributed to iron limitation in the Southern Ocean (Martin 1990) or excessive stratification of the summer mixed layer in coarse resolution ocean models (Aumont and Bopp 2006). The PlankTOM10 model produces a more realistic balance of lower chla concentration in the Southern Hemisphere compared to the Northern Hemisphere than the PlankTOM6 model (Figure S6). Role of macrozooplankton in ecosystem functioning To explore the cause of the difference between PlankTOM10 and PlankTOM6, we constructed two sets of two additional experiments each (Table S4). In the first set of experiments, we removed the macrozooplankton PFT from PlankTOM10, and in parallel we added the macrozooplankton PFT to PlankTOM6. These two experiments show that macrozooplankton play a pivotal role in determining the relative average concentrations of chla in the Northern versus Southern hemisphere (Pacific Ocean): removing macrozooplankton from PlankTOM10 reduces the North/South ratio of average chla from 1.9 to 1.3 (same as PlankTOM6), which is much lower than the observed ratio of 2.2. In the same way, including macrozooplankton in PlankTOM6 increases the North/South ratio from 1.3 to 1.8, which is very close to the PlankTOM10 ratio of 1.9. In the second set of experiments, we test if the importance of macrozooplankton is due to its growth characteristics or to the addition of a foodweb compartment. To test this, we parameterised macrozooplankton using the same vital rates as mesozooplankton in PlankTOM10, and in parallel we parameterised mesozooplankton using the same vital rates as macrozooplankton in PlankTOM6. On the one hand, the North/South ratio of average chla increases from 1.3 to 2.4 when mesozooplankton are parameterised as macrozooplankton in PlankTOM6, and on the other hand, decreases from 1.3 to 1.0 when macrozooplankton are parameterised as mesozooplankton in PlankTOM10. These results indicate that the North/South contrast is determined by the characteristics of macrozooplankton, especially their low growth rates and long life times, and not by the addition of a food-web compartment. Grazing by macrozooplankton adds important ecosystem fluxes to the model. To contrast the effect of macrozooplankton on average chla in the two hemispheres, we analysed the fluxes and biomasses in the North and South Pacific, where temperature and export production are similar but the ratio of average chla is 1.7 (Figure S7). In the North Pacific, the macrozooplankton biomass is large and the grazing on other zooplankton is consequently also large. In the South Pacific, the macrozooplankton biomass is 6.4 times lower than in the North, which reduces the grazing on other zooplankton correspondingly. This lower grazing on zooplankton results in higher grazing rates of meso- and protozooplankton on phytoplankton biomass, which reduces the surface chla concentration. The presence of macrozooplankton thus accelerates the recycling of organic matter in surface waters, which increases primary production. These results raise the importance of top-down control on chla distribution, and suggest that food-web structure and not Fe limitation keeps the low chla concentration in the Southern Ocean. This is supported by the fact that although Fe is lower in the Southern Ocean than elsewhere, it is generally above the half-saturation value for growth of most organisms and thus not necessarily a limiting factor. This is not in contradiction to the results of Fe fertilisation experiments (Boyd et al. 2007), because as long as the Fe is not at its optimal concentration, additional Fe will trigger further growth. If macrozooplankton control plankton biomasses in the Southern Ocean, the potential of Fe fertilisation for climate control is quite limited. Importance of PFT diversity for ecosystem supporting services We explored the role of phytoplankton PFT (pPFT) diversity for ecosystem supporting services (i.e. primary, secondary and export production) through controlled sensitivity experiments. In two sets of experiments, we added pPFTs one at the time on top of, first, a model that initially included two pPFTs (diatoms and picophytoplankton), or second, a model that included five pPFTs (i.e. the six in PlankTOM10 minus the one added). The effect of each pPFT on primary production is far greater when pPFT diversity is low, with primary production increasing by 1.7 to 6.6 PgC yr-1 depending on the pPFT, compared to 0.5 to 2.7 PgC yr-1 when the pPFT diversity is high (Figure S8 left and middle, respectively). This result suggests that the addition of new pPFTs to the model, on top of the six already represented in PlankTOM10, would likely have limited impact on primary production. Such a saturation effect has been observed for terrestrial grassland (Hector et al. Nature 2007). In an additional sensitivity experiment, we represented all the pPFTs except diatoms using the same set of parameters (Figure S8 right). The results indicate that pPFT diversity did not in itself lead to high primary production, which can be higher if all pPFTs have high growth rates such as those of Phaeocystis or coccolithophores. Hence although pPFT diversity improves the representation of surface chla (see above), their current geographic distribution is a function of the competition between groups and does not lead to an optimal use of resources. Finally we explored the role of food-web structure for ecosystem services by successively increasing and decreasing the growth rate of pPFTs, bacteria, protozooplankton, mesozooplankton, and macrozooplankton by factors up to four (Figure 5). These controlled experiments test the response to a range of processes that could occur in the ocean in response to changes in nutrient supply from the mid-depth ocean or atmospheric dust deposition, warming, ocean acidification, top-down grazing control, viral infections, invasion of alien species, or ecosystem shifts towards more or less competitive species. Primary production responds almost linearly to perturbations in growth rate of all groups except mesozooplankton, and is most influenced by the growth of the smaller PFTs (pPFTs, bacteria, and protozooplankton). Primary production is also affected by mesozooplankton growth, i.e. it decreases when mesozooplankton growth is both decreased and increased, which indicates that mesozooplankton growth is near its optimal value in PlankTOM10. Finally, primary production responds positively to increasing macrozooplankton growth, which is consistent with their effect on phytoplankton biomass and production proposed above. Secondary production responds most strongly to changes in bacteria. For the PFTs that have strong effects on primary production and comparatively smaller effects on secondary production, their strong effects on the former seem to be dampened by ecosystem feedbacks that reduce the ecological efficiency of the food web. This could be interpreted as natural resilience of pelagic ecosystems, created by their dynamics, to perturbations in the ocean’s environment. Panel (c) of Fig. 5, i.e. ecological efficiency, is referred to only once in the Discussion. I suggest to either thoroughly discuss it, or remove it. I favour removal. Export production responds non-linearly to changes in growth rates for all sets of experiments suggesting the presence of feedbacks (stabilising or amplifying) mechanisms in lower food-web ecosystems. Export production shows higher values in perturbed than unperturbed runs for only two of the five PFTs, i.e. phytoplankton and bacteria. Hence, export production is largely controlled by the competition between the growth of phytoplankton and that of bacteria. Changes in zooplankton growth rates either leave export unchanged, or lead to reduced export because the change in zooplankton grazing on primary production is compensated by changes in the downward fluxes of faecal pellets and other fast sinking organic materials. In particular, reduction in mesozooplankton growth by a factor larger than 2.0 leads to fast non-linear reduction in export because as mesozooplankton growth decreases, primary production also decreases because of the decreased pressure on protozooplankton, which leads to a decrease in the e-ratio that creates an amplification effect. In contrast, when protozooplankton growth decreases, primary production increases but the e-ratio decreases because of more recycling, creating a dampening effect. Inreasing the macrozooplankton growth rate decreases export production, which is consistent with their enhancement of recycling proposed above, and inreases the fraction of primary production that is exporte. However, their influence is small because the grazing of macrozooplankton is also small. Increased pPFT growth leads to increased export, but not as much as expected from the increased primary production alone because some of the pPFT growth is based on recycled nutrients. Finally, changes in bacterial growth rates above a factor of two lead to strong non-linear feedbacks, which quickly amplify the change in export. #CORRINE: I find that discussing together export production and the e-ratio creates much confusion. I suggest to discuss these in two different, successive pragraphs, because the e-ratio depends on both primary and export production. Interpretating the e-ratio will prove to be quite difficult. I am wondering if we should not be remove the e-ratio from our general-redearship paper. To assess the robustness of these results, we modified the PlankTOM10 model parameters to improve the covariance between mesozooplankton and chla by increasing the mesozooplankton mortality and its temperature dependence (see the legends of Figures S4 and S9). Conclusions on the linear response of primary production and non-linear response of export to changes in growth rates are robust to this experiment, although the position of the thresholds varied (Figure S9). #CORRINE: I think that there must be a brief discussion, in this Section or in the Conclusions, on macrozooplankton. We must bring together the major role given to macrozooplankton in the previous Section with the results of the sensitivity analysis. The latter are fully consistent with the previous Section for primary and export production. Conclusions The presence of feedbacks between marine export production and PFT growth is of critical importance because ocean’s export is involved in the long-term regulation of climate [16]. Shifts in ecosystems and the disappearance of species have occurred in the past (Erba 2004, Caswell et al. 2009, Bucefalo Palliani et al. 2002), during extensive ocean deoxygenation either regionally (Diaz J, Env Qual 2001) or globally (Jenkyns, H. C. (2010). Geochem. Geophys. Geosystems). Such shifts could occur in the future from a range of perturbations. Further work is needed to better identify the processes that can trigger nonlinear and amplifying feedbacks, and to assess the stability of the current marine ecosystem. This would require progress to advance our quantitative understanding by: (1) improving the data on rates of PFTs, in particular on the half-saturation values and food preferences, and on PFT-specific biomass, (2) improving our understanding of ecosystem feedbacks, possibly by undertaking manipulative experiments in the ocean or the laboratory, and (3) developing and testing a variety of models of ecosystem dynamics, including self-assembling models (Follows et al) and biogeochemical food-web models that include large organisms such as fish and marine mammals (deYoung et al. 2004). Identifying feedbacks and possible thresholds in marine ecosystems is crucial to determine the limits of dangerous climate change. Acknowledgments The design of the DGOM was developed through a series of seven international workshops funded in part by the Max-Planck Institute for Biogeochemistry, and hosted by the Villefranche Oceanography Laboratory, France. We thank Stéphane Pesant for support with the data compilations. This work benefited from funding by the European Union EUR-OCEANS, GREENCYCLES, Carbo-Ocean and Carbo-Change projects and the UK-NERC QUEST programme. We thank the NEMO team (Nucleus for European Modelling of the Ocean) for support to the physical model. Model simulations were run on the Escluster of the University of East Anglia. Table 1. Growth rates of PFTs at 0 and 10°C (µ0 and µ20), and factors of rate increase for 10°C rise (Q10). The uncertainty in μ0 and Q10 represents ±1 standard deviation from the optimal parameter values, in the parameter space (Dieter, a ref?). μ0 Q10 μ20 number of data 0.045 ± 0.06 1.84 ± 2.73 0.15 36 Picophytoplanktonb 0.27 ± 0.06 1.79 ± 0.18 0.87 152 Coccolithophores 0.51 ± 0.17 1.41 ± 0.26 1.01 221 Mixed phytoplanktonc 0.35 ± 0.05 1.57 ± 0.12 0.86 95 LaRoche and Breitbarth 2005g Agawin 1998; Johnson 2006; Moore 2005 Buitenhuis et al. 2008; Larsen et al., in prep Bissinger et al. 2008g Phaeocystis 0.67 ± 0.07 1.66 ± 0.12 1.85 68 Schoemann et al. 2005g Diatoms 0.41 ± 0.02 2.05 ± 0.07 1.72 569 Sarthou et al. 2005g 0.58 ± 0.02 1.45 1.22 2260 Rivkin and Legendre 2001g Protozooplanktond 0.32 ± 0.02 1.79 1.03 1079 Buitenhuis et al. 2010g Mesozooplanktone 0.30 ± 0.02 1.28 0.49 2745 Buitenhuis et al. 2006g PFT Autotrophs N2-fixersa Heterotrophs Bacteria main references Macrozooplanktonf 0.06 ± 0.01 1.80 0.19 254 Hirst and Bunker 2003g aTrichodesmium and N -fixing unicellular prokaryotes 2 bPico-eukaryotes and non N -fixing photosynthetic bacteria such as Synechococcus and Prochlorococcus 2 cAutotrophic dinoflagellates and Chrysophyceae dHeterotrophic flagellates and ciliates eCopepods and amphipods fEuphausids and pteropods gThese references include syntheses of data from other papers Notes: Note 1: The exponential fit to growth observations was obtained by optimising the relation: μT = μ0 * XT where T and μT are the observed temperature and associated growth rate, μ0 is the growth at 0°C, and X10 is the Q10 of growth. The parameter values for μ0 and Q10 were estimated based on the pair of parameters that produced the smallest least square fit of the function (μT-μobsT)/μobsT. Normalisation to observations was used here to ensure a good fit of μT in cold waters where growth rates are low. The growth rate parameters estimated with this method are well constrained for nine of the ten PFTs (Table 1). There are insufficient data to provide strong constraints on the growth rates of N2-fixers, and the uncertainty in their parameters is large (Table 1). However the growth of N2-fixers is well below that of other phytoplankton PFTs (Figure 1), and even large errors in the parameters still produce μT below that of other PFTs. The growth rate of coccolithophores was artificially high at cold temperatures due to the presence of high growth data around 20°C and the absence of data below 5°C. We reduced the fitted growth rate of coccolithophores linearly to 0 below 4°C to match the observations at cold temperatures. The growth rate for all phytoplankton combined is comparable to that estimated by [17]. Appendix A: Model description PlankTOM10 represents 3 detrital pools: Dissolved Organic Matter (DOM), Small Particulate Organic Matter (POM) and Large Particulate Organic Matter (GOM). The sinking speed of GOM is based on the mineral (ballast) content of particles [18]. POM sinks at a constant speed of 3 m/s. The model includes full cycles of C, O2, and P, which are fixed and released by biological processes following a constant ratio of 122:161:1, a full cycle of Si [16], and simplified cycles for Fe and N as detailed below. CO2 and O2 are exchanged with the atmosphere using the gas exchange formulation of [19]. The cycle of Fe is represented as in [5]. Fe is deposited with dust particles using the monthly fields of [20], the Fe content of dust is 3.5%, and Fe has a solubility of 1%. Fe is also transported through river fluxes following the outflow scheme of [14] with 99% of sedimentation in estuaries. Dissolved Inorganic Nitrogen (DIN) is included in the model as the sum of nitrate and amonium. The N:P ratio of organic processes is set to the Redfield ratio of 16:1, except for N2-fixers which can use N2 and thus have access to unlimited N source from the atmosphere. Phytoplankton PFTs are limited by light and nutrients (P, N, Si, and Fe). Initial values for the half saturation of growth rates for P (kP) and N (kN) were based on the following observations: For N2fixers, coccolithophores and diatoms, the half-saturation values of growth were computed by multiplying the half-saturation values of uptake reported in [11, 21, 22] by 1/3 [23]. For picophytoplankton, reported values covered three orders of magnitude. We opted to assign low halfsaturation values as picophytoplankton is observed to grow even under very low nutrient conditions. We assigned the same value for mixed phytoplankton as for diatoms. For Phaeocystis, we used k values that characterise colonies [24]. The selected set of parameter values were adjusted to reproduce the co-variances between chla and N and P distributions, which show more limitation from N at low chla concentrations, and for P at intermediate chla concentrations (Figure 2). Final parameter values are reported in Table S1 and S2 and in the supplementary material. The Fe uptake was computed with a quota model based on an extension of the work of [23] by [25]. The Fe uptake by phytoplankton PFTs is regulated by the light conditions. Three parameters are needed: the minimum, maximum and optimal Fe quotas. The minimum and maximum quotas were set at the same value of 2.5 and 20 μmol Fe/mol C for all PFTs. The optimal quota was set to the minimum Quota + 2*μ20max based on [Erik/Richard G., please include comment here]. The half saturation of Fe uptake (kFe) was reported as lower than other phytoplankton for picophytoplankton [26] and higher for N2-fixers [21] and diatoms [11]. Intermediate values for kFe were reported for the other PFTs [24, 27]. The selected set of parameter values after adjustments in kFe produces no systematic co-variance between chla and Fe, as observed (Figure 2), although it shows a peak at 0.3 nmol/L whereas the observations peak at 1.1 nmol/L. Light limitation is constrained by the slope of the productivity-irradiance (α) curve and the maximum Chl/C ratio (θ; see Supplementary information for the full set of model equations). We could not distinguish PFT-specific values for α [28] and used a mean value of 1.0 for all PFTs. Observaed θ for diatoms was systematically higher than that of other PFTs [28]. For other groups, insufficient observations were directly available. We fitted the observations [28] for θ to the μ0max presented in the same paper. The fit showed θ increasing with growth rate. We thus used a θ higher than average for Phaeocystis and diatoms, and θ lower than average for N2-fixers . The half-saturation growth rate values for zooplankton PFTs (kFood) was initially set to a constant value of 20 μmol for zooplankton and 60 μmol for bacteria based on the analysis of [29], and subsequently adjusted to reproduce the chla-zooplankton co-variance (Figure 2). The adjustments allowed to reproduce the steeper co-variance between chla and protozooplankton and the absence of co-variance between chla and macrozooplankton. The ecological behaviour of macrozooplankton was taken into account by parameter adjustments: horizontal advection of macrozooplankton was halved when the sea depth was <600 m to account for the motility of macrozooplankton and their capacity to seek shelter and escape grazing by top predators; and based on observations [12], their growth rates were increased by a factor of 100 when the fraction of sea covered by ice exceeded 0.1 in the summer to account for the improved survival rate of larvae when ice is present. The ecological adjustments to macrozooplankton only changed local concentrations but did not affect their overall patterns. It was not possible to reproduce simultaneously the co-variance patterns for all zooplankton, which suggests that either the initial parameters were far from observations or that additional ecological processes play important roles (e.g. vertical migration). PlankTOM10 is coupled online to the NEMO version 3.1 (NEMOv3.1). NEMOv3.1 is an Ocean General Circulation Model (OGCM). We used the global ORCA configuration [30], which has a resolution of 2° of longitude and a mean resolution of 1.5° of latitude, with enhanced resolution to 0.3° in the tropics and at high latitudes. The model resolves 30 vertical levels, with 10 m depth in the top 100 m. NEMOv3.1 calculates vertical diffusion explicitly and represents eddy mixing using the parameterisation of (Gent and McWilliams 1992). NEMOv3.1 is coupled to a dynamicthermodynamic sea-ice model (Fichefet and Morales-Maqueda, 1999). The model is initialised from observations for DIC and alkalinity from Key et al. (2004), O2 and nutrients from (Garcia et al., 2006a;2006b,), temperature and salinity from the World Ocean Atlas (Levitus et al. 2005). The PFT compartments equilibrate very rapidly (within 3 years) and were simply initialised with results from previous model simulations. The model is forced by winds and precipitations from the ECMWF Interim reanalysis (ref). It calculates heat fluxes and evaporation using a bulk formulation based on the difference between ECMWF’s surface air temperature and the sea surface temperature produced by the model. Mean daily forcing is imposed on the model for 1989-2009. The results presented here are averaged over 2005-2009. A full set of equations and parameters is provided in the supplementary material. Figure captions Figure 1. Specific growth for 10 Plankton Functional Types as a function of temperature. References are provided in Table 1 and the Supplementary data. Figure 2. Covariance between total chla and limiting nutrients (left) and biomass of heterotrophic plankton (right). Chlorophyll data from SeaWiFS are the same in each plot, and are averages of 19982009. The NO3- and PO43- fields are from the World Ocean Atlas [31]. Fe concentration is updated from [5]. The proto- and mesozooplankton biomass data are from [18, 25], respectively. The macrozooplankton biomass data are from Atkinson et al. (2005). The data were binned into intervals of approximately the same numbers of observations. The median is shown in black, and the grey shading represents the 25-75% data range. Figure 3. Annual mean surface chla (mgChl m-3) at the ocean surface from (top) SeaWiFS, (middle) PlankTOM10, and (bottom) PlankTOM6. All datasets are averaged over the 1998-2009 time period. Figure 4. Annual mean surface biomass for the 10 Plankton Functional Types as reproduced by the PlankTOM10 model (μmol L-1). Figure 5. Effects of changes in growth rates of PFTs of different food-web compartments on marine ecosystem supporting services, from sensitivity experiments. The growth rates of phytoplankton PFTs (pPFT2), bacteria, protozooplankton mesozooplankton, and macrozooplankton were increased or decreased one at the time by a factor up to 4. For the pPFT experiments, the growth rate of all pPFTs was changed by the same proportional amount. The x-axis is shown in log scale so that an increase by factor 2 (0.3) is equally distant from 0 (i.e. no change) as a reduction by half (-0.3). Results are shown for (a) primary production, (b) secondary production, (c) export production, (d) ecological efficiency (the ratio or secondary to primary production), and (e) e-ratio (the ratio of export to primary production). Each point results from one sensitivity experiment, and is the average of three years (1992-1994). References 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. Worm, B., et al., Impacts of biodiversity loss on ocean ecosystem services. Science, 2006. 314(5800): p. 787-790. MaierReimer, E., U. Mikolajewicz, and A. Winguth, Future ocean uptake of CO2: Interaction between ocean circulation and biology. Climate Dynamics, 1996. 12(10): p. 711-721. Le Quere, C., et al., Ecosystem dynamics based on plankton functional types for global ocean biogeochemistry models. Global Change Biology, 2005. 11(11): p. 2016-2040. Hood, R.R., et al., Pelagic functional group modeling: Progress, challenges and prospects. Deep-Sea Research Part Ii-Topical Studies in Oceanography, 2006. 53(5-7): p. 459-512. Aumont, O. and L. Bopp, Globalizing results from ocean in situ iron fertilization studies. Global Biogeochemical Cycles, 2006. 20(2). Bopp, L., et al., Dust impact on marine biota and atmospheric CO2 in glacial periods. Geochimica Et Cosmochimica Acta, 2002. 66(15A): p. A91-A91. Gregg, W.W., et al., Phytoplankton and iron: validation of a global threedimensional ocean biogeochemical model. Deep-Sea Research Part Ii-Topical Studies in Oceanography, 2003. 50(22-26): p. 3143-3169. Moore, L.R., R. Goericke, and S.W. Chisholm, Comparative Physiology of Synechococcus and Prochlorococcus - Influence of Light and Temperature on Growth, Pigments, Fluorescence and Absorptive Properties. Marine EcologyProgress Series, 1995. 116(1-3): p. 259-275. Buitenhuis, E., et al., Biogeochemical fluxes through mesozooplankton. Global Biogeochemical Cycles, 2006. 20(2). Buitenhuis, E.T., et al., Biogeochemical fluxes through microzooplankton. Global Biogeochemical Cycles, 2010. 24. Sarthou, G., et al., Growth physiology and fate of diatoms in the ocean: a review. Journal of Sea Research, 2005. 53(1-2): p. 25-42. Wiedenmann, J., K.A. Cresswell, and M. Mangel, Connecting recruitment of Antarctic krill and sea ice. Limnology and Oceanography, 2009. 54(3): p. 799-811. Schneider, B., et al., Climate-induced interannual variability of marine primary and export production in three global coupled climate carbon cycle models. Biogeosciences, 2008. 5(2): p. 597-614. da Cunha, L.C., et al., Potential impact of changes in river nutrient supply on global ocean biogeochemistry. Global Biogeochemical Cycles, 2007. 21(4). Giraud, X., C. Le Quere, and L.C. da Cunha, Importance of coastal nutrient supply for global ocean biogeochemistry. Global Biogeochemical Cycles, 2008. 22(2). Maierreimer, E., Geochemical Cycles in an Ocean General-Circulation Model Preindustrial Tracer Distributions. Global Biogeochemical Cycles, 1993. 7(3): p. 645-677. Bissinger, J.E., et al., Predicting marine phytoplankton maximum growth rates from temperature: Improving on the Eppley curve using quantile regression. Limnology and Oceanography, 2008. 53(2): p. 487-493. Buitenhuis, E., et al., Trends in inorganic and organic carbon in a bloom of Emiliania huxleyi in the North Sea. Marine Ecology-Progress Series, 1996. 143(1-3): p. 271-282. Wanninkhof, R., Relationship between wind speed and gas exchange over the ocean. Journal of geophysical research 1992. 97(C5): p. 7373-7382. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 1 Jickells, T.D., et al., Global iron connections between desert dust, ocean biogeochemistry, and climate. Science, 2005. 308(5718): p. 67-71. LaRoche, J. and E. Breitbarth, Importance of the diazotrophs as a source of new nitrogen in the ocean. Journal of Sea Research, 2005. 53(1-2): p. 67-91. Riegman, R., I.A. Flameling, and A.A.M. Noordeloos, Size-fractionated uptake of ammonium, nitrate and urea and phytoplankton growth in the North Sea during spring 1994. Marine Ecology-Progress Series, 1998. 173: p. 85-94. Geider, R.J., H.L. MacIntyre, and T.M. Kana, A dynamic regulatory model of phytoplanktonic acclimation to light, nutrients, and temperature. Limnology and Oceanography, 1998. 43(4): p. 679-694. Schoemann, V., et al., Phaeocystis blooms in the global ocean and their controlling mechanisms: a review. Journal of Sea Research, 2005. 53(1-2): p. 43-66. Buitenhuis, E.T. and R.J. Geider, A model of phytoplankton acclimation to iron-light colimitation. Limnology and Oceanography, 2010. 55(2): p. 714724. Timmermans, K.R., et al., Physiological responses of three species of marine pico-phytoplankton to ammonium, phosphate, iron and light limitation. Journal of Sea Research, 2005. 53(1-2): p. 109-120. Le Vu, B., La biocalcification dans l'océan actuel à travers l'organisme modèle Emiliania huxleyi, 2005, Universite Pierre et Marie Curie: Paris. Geider, R.J., H.L. MacIntyre, and T.M. Kana, Dynamic model of phytoplankton growth and acclimation: Responses of the balanced growth rate and the chlorophyll a:carbon ratio to light, nutrient-limitation and temperature. Marine Ecology-Progress Series, 1997. 148(1-3): p. 187-200. Hansen, P.J., P.K. Bjornsen, and B.W. Hansen, Zooplankton grazing and growth: Scaling within the 2-2,000-mu m body size range. Limnology and Oceanography, 1997. 42(4): p. 687-704. Madec, G. and M. Imbard, A global ocean mesh to overcome the North Pole singularity. Climate Dynamics, 1996. 12(6): p. 381-388. Garcia, H.E., R. A. Locarnini, T. P. Boyer, and J. I. Antonov, World Ocean Atlas 2005, Volume 4: Nutrients (phosphate, nitrate, silicate), in NOAA Atlas NESDIS S. Levitus, Editor 2006, U.S. Government Printing Office: Washington D.C. . p. 396. See full model description and evaluation in Supporting Online Material.