intro to analytical chem test
advertisement

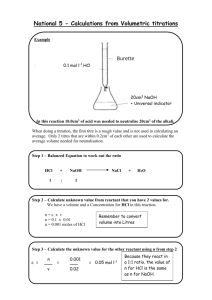
Unit 3 Chemistry Name:_______________ Introduction to Analytical Chemistry Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. In the following reaction, 6 mol of aluminium is mixed with 6 mol of oxygen: 4Al(s) + 3O2(g) 2Al2O3(s) What amount of aluminium oxide will be produced from this reaction? A. 1 mol B. 2 mol C. 3 mol D. 4 mol 2. Which one of the following is a triprotic Lowry-BrØnsted acid? A. CH3COOH B. H3PO4 C. H2CO3 D. NH3 3. The concentration of hydroxide ions in limewater, Ca(OH)2, of concentration 0.01 mol L-1 is: A. 10-12 mol L-1 B. 0.005 mol L-1 C. 0.01 mol L-1 D. 0.02 mol L-1 4. The concentration of hydroxide ions in HCl of concentration 0.01 mol L-1 is: A. 10-12 mol L-1 B. 0.01 mol L-1 C. 2 mol L-1 D. 12 mol L-1 5. Which one of the following equations does NOT represent a Lowry-BrØnsted acid-base reaction? A. NH3(aq) + H2O(l) NH4+(aq) + OH-(aq) B. 2HCl(aq) + Mg(s) MgCl2(aq) + H2(g) C. H2SO4(aq) + MgCO3(s) MgSO4(aq) + H2O(l) + CO2(g) D. HSO4-(aq) + H2O(l) SO42-(aq) + H3O+(aq) 6. The pH of a solution of HNO3 of concentration 1 mol L-1 is: A. 0 B. 1 C. -1 D. 14 7. Which statement about water in the following half-equation is CORRECT? 2H2O(l) + 2e- A. B. C. D. H2(g) + 2OH-(aq) H2O has acted as a reductant only. H2O has acted as an oxidant only. H2O has acted both as an oxidant and a base. H2O has self-ionised. 8. Which one of the following half-equations is correctly balanced? A. Cl2(g) + e- Cl-(aq) B. Al3+(aq) Al(s) + 3eC. Fe3+(aq) + e- Fe2+(aq) D. NO3-(aq) + 2H+(aq) NO2(g) + H2O(l) + e- 9. The lead-based pigments in old paintings have often deteriorated into black lead(II) sulfide, PbS, due to exposure to hydrogen sulfide in the air. To restore the original colour, the PbS is treated with hydrogen peroxide solution, H2O2. The net equation for the reaction is: PbS(s) + 4H2O2(aq) PbSO4(s) + 4H2O(l) In this reaction, in which one of the following capacities is the hydrogen peroxide acting? A. Acid B. Precipitating agent C. Reductant D. Oxidant 10. Which one of the following solutions would LOWER the pH if added to 50 mL of a certain solution of pH of 4? A. 50 mL water B. 50 mL NaOH solution of concentration 4 mol L-1 C. 50 mL HCl of concentration 0.0001 mol L-1 D. 50 mL HCl of concentration 4 mol L-1 11. One substance that can cause acid rain is nitrogen dioxide gas, NO2. What is the mass, in grams, of one molecule of nitrogen dioxide? A. B. C. 46.0 × 6.02 × 1023 D. 46.0 12. If 50.0 mL of HCl of concentration 0.300 mol L-1 is added to 50.0 mL of NaOH of concentration 0.100 mol L-1, what will be the pH of the final solution? A. 0.100 B. 1.00 C. 7.00 D. 13.0 13. Ozone in the air at ground level presents a serious pollution problem. A 1.0 m3 sample of air collected at 100 kPa and 24 °C was found to contain 38.5 mL of ozone. The amount of ozone gas in the sample would be closest to: A. 0.001 56 mol B. 0.0193 mol C. 1.56 mol D. 19.3 mol 14. The formula NOx represents the family of oxides of nitrogen. One oxide was found to contain 30.4 % nitrogen by mass. What was the empirical formula of the oxide? A. N2O B. N2O4 C. NO2 D. NO 15. To deduce the molecular formula of a certain hydrocarbon, an experiment was conducted in which lithium hydroxide was used to absorb carbon dioxide produced in the combustion of the hydrocarbon. Lithium hydroxide reacts with the carbon dioxide according to this equation: LiOH(s) + CO2(g) LiHCO3(aq) What is the maximum volume of CO2 that can be absorbed at SLC by 10 g of lithium hydroxide? A. 10 mL B. 98 mL C. 9.8 L D. 10 L 16. If 0.5 mol of C6H14 is burnt completely in air at 1000 °C and 760 mmHg, what will be the total volume of the gas products? A. 20 L B. 300 L C. 600 L D. 700 L 17. Two gas syringes each contain the same volume of gas at 25 °C. One of these gas syringes contains 0.010 g neon gas at 100 kPa, and the other contains 0.010 g propane gas, C3H8. The pressure exerted by the propane gas on its gas syringe will be: A. 46 kPa B. 100 kPa C. 200 kPa D. 220 kPa 18. Ammonia gas can react with oxygen gas to produce nitrogen gas and steam, according to this equation: 4NH3(g) + 3O2(g) 2N2(g) + 6H2O(g) If 2.0 L of ammonia is mixed with a certain volume of excess oxygen and the final volume is 6.0 L, with all gases measured at the same temperature and pressure, the volume of oxygen originally added must be: A. 1.5 L B. 2.0 L C. 3.5 L D. 4.0 L 19. 1 L of a certain hydrocarbon was burnt in excess air at 500 °C and 1 atm pressure. The carbon dioxide and steam produced in this reaction were found to occupy 5 L and 6 L respectively when measured at 500 °C and 1 atm pressure. What was the molecular formula of the hydrocarbon? A. C3H8 B. C5H6 C. C5H12 D. C6H12 20. Acidified potassium dichromate, K2Cr2O7, reacts with ethanol, C2H5OH, according to the following ionic equation: 2Cr2O72-(aq) + 16H+(aq) + 3C2H5OH(aq) 4Cr3+(aq) + 3CH3COOH(aq) + 11H2O(l) A 20.00 mL aliquot of a standard solution of acidified potassium dichromate, of concentration 0.07500 mol L-1, was reacted with a solution of ethanol. The mean volume of the ethanol solution required for the titration was 25.32 mL. The concentration of ethanol in the solution, in mol L-1, is: A. 0.03949 B. 0.05917 C. 0.08886 D. 0.1185