Supplemental Digital Content 3. Supplemental Table1: Summary of
advertisement
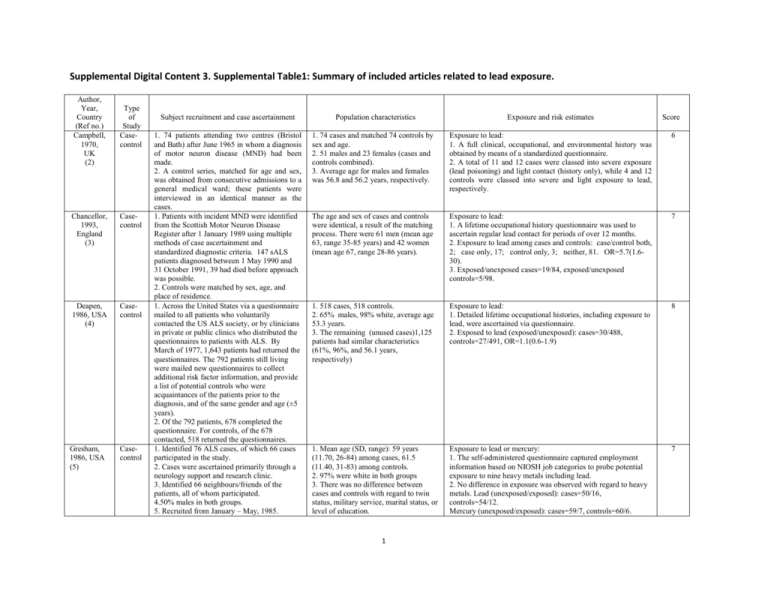
Supplemental Digital Content 3. Supplemental Table1: Summary of included articles related to lead exposure. Author, Year, Country (Ref no.) Campbell, 1970, UK (2) Type of Study Casecontrol Chancellor, 1993, England (3) Casecontrol Deapen, 1986, USA (4) Casecontrol Gresham, 1986, USA (5) Casecontrol Subject recruitment and case ascertainment 1. 74 patients attending two centres (Bristol and Bath) after June 1965 in whom a diagnosis of motor neuron disease (MND) had been made. 2. A control series, matched for age and sex, was obtained from consecutive admissions to a general medical ward; these patients were interviewed in an identical manner as the cases. 1. Patients with incident MND were identified from the Scottish Motor Neuron Disease Register after 1 January 1989 using multiple methods of case ascertainment and standardized diagnostic criteria. 147 sALS patients diagnosed between 1 May 1990 and 31 October 1991, 39 had died before approach was possible. 2. Controls were matched by sex, age, and place of residence. 1. Across the United States via a questionnaire mailed to all patients who voluntarily contacted the US ALS society, or by clinicians in private or public clinics who distributed the questionnaires to patients with ALS. By March of 1977, 1,643 patients had returned the questionnaires. The 792 patients still living were mailed new questionnaires to collect additional risk factor information, and provide a list of potential controls who were acquaintances of the patients prior to the diagnosis, and of the same gender and age (±5 years). 2. Of the 792 patients, 678 completed the questionnaire. For controls, of the 678 contacted, 518 returned the questionnaires. 1. Identified 76 ALS cases, of which 66 cases participated in the study. 2. Cases were ascertained primarily through a neurology support and research clinic. 3. Identified 66 neighbours/friends of the patients, all of whom participated. 4.50% males in both groups. 5. Recruited from January – May, 1985. Population characteristics Exposure and risk estimates Score 1. 74 cases and matched 74 controls by sex and age. 2. 51 males and 23 females (cases and controls combined). 3. Average age for males and females was 56.8 and 56.2 years, respectively. Exposure to lead: 1. A full clinical, occupational, and environmental history was obtained by means of a standardized questionnaire. 2. A total of 11 and 12 cases were classed into severe exposure (lead poisoning) and light contact (history only), while 4 and 12 controls were classed into severe and light exposure to lead, respectively. 6 The age and sex of cases and controls were identical, a result of the matching process. There were 61 men (mean age 63, range 35-85 years) and 42 women (mean age 67, range 28-86 years). Exposure to lead: 1. A lifetime occupational history questionnaire was used to ascertain regular lead contact for periods of over 12 months. 2. Exposure to lead among cases and controls: case/control both, 2; case only, 17; control only, 3; neither, 81. OR=5.7(1.630). 3. Exposed/unexposed cases=19/84, exposed/unexposed controls=5/98. 7 1. 518 cases, 518 controls. 2. 65% males, 98% white, average age 53.3 years. 3. The remaining (unused cases)1,125 patients had similar characteristics (61%, 96%, and 56.1 years, respectively) Exposure to lead: 1. Detailed lifetime occupational histories, including exposure to lead, were ascertained via questionnaire. 2. Exposed to lead (exposed/unexposed): cases=30/488, controls=27/491, OR=1.1(0.6-1.9) 8 1. Mean age (SD, range): 59 years (11.70, 26-84) among cases, 61.5 (11.40, 31-83) among controls. 2. 97% were white in both groups 3. There was no difference between cases and controls with regard to twin status, military service, marital status, or level of education. Exposure to lead or mercury: 1. The self-administered questionnaire captured employment information based on NIOSH job categories to probe potential exposure to nine heavy metals including lead. 2. No difference in exposure was observed with regard to heavy metals. Lead (unexposed/exposed): cases=50/16, controls=54/12. Mercury (unexposed/exposed): cases=59/7, controls=60/6. 7 1 4. Did not mention smoking and alcohol use, or lifestyle related factors. Kamel, 2002, USA (6) Casecontrol McGuire, 1997, USA (7) Casecontrol PierceRuhland, 1981, USA (8) Casecontrol 1. All study participants (cases and controls) were recruited between 1993 and 1996. 2. Cases were required to live in New England for at least half of the year, be mentally competent, and speak English. 3. 71% of eligible cases participated in the study (n = 111). About 85% of the cases were enrolled within 1 year after diagnosis and the remainder within 2 years. 4. Population controls with no neurological diseases were identified through random telephone screening and matched on sex, age (30–55, 56–65, and 66–80 years) and region. 354 eligible controls were contacted, 270 (76%) were enrolled, and 256 completed the entire questionnaire. 1. Recruited during the period 1990-1994. 2. ALS cases aged l8 or older years who were newly diagnosed with ALS in western Washington State were identified through a surveillance system; cases who did not have a telephone or did not speak English were excluded. 3. 174 of 180 eligible cases agreed to participate. 4.Two controls matched to each case according to sex and age (±5 years) were identified from the study counties using random telephone dialling; for controls over 65 years of age, Medicare eligibility lists for the target counties were used. The overall response rate was 75%. No differences were noted cases and controls with regard to age, sex ratio, and level of education. 1. 88 living patients were identified from hospital records, of which 80 participated the study. 2. Patients’ friends of the same sex and age matched within 5 years were suggested by the patients. 80 controls were contacted, 78 controls participated. 1. 80 cases: 53 men, 27 women 2. 78 controls: 52 men, 26 women. 3. Mean age at interview (62 years) was the same in both groups. . 1. Cases=174, controls=348. 2. Men: cases=95, controls=190, accounting for 54.6% of participants. . 3. White: cases=164, controls=329,, accounting for 94.3% and 94.5% of cases and controls, respectively. 4. Although the average age was not specified, the age range was similar among cases and controls.. 5. No differences in marital status were apparent. 6. Differences in education (low/high) were noted: cases=83/91, controls=124/224. 2 3. Exposure to lead/mercury in case-control pairs (cases/controls, +=yes, -=no): lead/mercury: +/+=6, +/-=13, -/+=6, -/-=8; OR=2.20(0.69-7.47). Lead: +/+=5, +/-= 12, -/+=6, -/-=10; OR=2.00 (0.63-7.00). Mercury: +/+= 2, +/-=6, -/+=4, -/-=21; OR=1.50 (0.31-8.26). Exposure to lead: 1. Occupational history information was obtained via interview. Duration of occupational exposure to lead was categorized into four levels (0, 1–399,400–1,999, and 2,000 days of exposure). Blood lead levels were also measured. 2. ALS risk was increased due to lead occupational exposure. Study participant exposures (unexposed/exposed) were: cases=67/35, controls=193/54. OR=1.9 (1.1-3.3). 3. A linear dose dependent increase in risk was observed: exp=0 days, OR=1 (reference category); exp=1-399 days, OR=1.6; exp=400-1999 days, OR=1.9; exp>=2000 days, OR=2.3, p for trend=0.02. 4. Mean (SD) blood lead levels were higher in ALS patients: cases=5.2(0.4), controls=3.4(0.4) µg/dl (P<0.05). Elevated blood lead levels were associated with an increased ALS r(up to 10 fold per µg/dl lead ). Exposure to lead -heavy metals: 1. Occupational histories and exposure to specific metals were ascertained by interview. Lead exposure information was based on self-reported exposure to specific metals or estimated via expert review of the occupational history. 1.1. Exposure to lead (self-reported, both sexes, exp/unexp): cases=21/153, controls=24/324, OR=1.9(1.0-3.6). 1.2. Exposure to lead (panel-estimated, both sexes, exp/unexp): cases=17/157, controls=31/317, OR=1.1(0.6-2.1). 2. Exposure to heavy metals: 2.1. Self-reported, both sexes, exp/unexp): cases=84/87, controls=139/209, OR=1.6(1.0-2.5). 2.2. Panel-estimated, both sexes, exp/unexp): cases=49/124, controls=82/266, OR=1.2(0.8-1.9). 2.3. Self-reported, males, exp/unexp): cases=63/31, controls=117/73, OR=1.2(0.7-2.1). 2.4. Panel-estimated, males, exp/unexp ): cases=45/49, controls=67/123, OR=1.5(0.9-2.6). 2.5. Self-reported, females, exp/unexp ): cases=21/56, controls=22/138, OR=2.3(1.1-4.7). 2.6. Panel-estimated, females, exp/unexp): cases=4/75, controls=15/143, OR=0.5(0.2-1.4). Exposure to lead and heavy metals 1. Lead exposure information was based on a telephone administered occupational questionnaire. Individuals with exposure durations less than or greater than one year were considered unexposed or exposed, respectively. 2. Lead (unexposed/exposed): cases=60/20, controls=67/11, OR=2.03(1.22-2.84),p=0.085. 13 10 6 Yu, 2014, USA (9) Casecontrol Binazzi, 2009, Italy (10) Casecontrol Morahan, 2006, Australia (11) Casecontrol Provinciali, 1990, Italy (12) Casecontrol RoelofsIverson, 1984, USA (13) Casecontrol Feychting , 2003, Sweden Cohort Study 1. ALS subjects were recruited through the University of Michigan ALS Clinic. Subjects were over 18 years of age, and had possible, probable, probable, or definite ALS according to the revised El Escorial criteria. 2. Age- and gender-matched controls were recruited through postings on a University. of Michigan website. Age was matched by decade 1. Recruited from July 17, 2005 to July 6, 2007. 77 patients, including 70 definite and 7 probable ALS cases; 44 spinal/ 32 bulbar. 2. Diagnosed based on EI Escorial criteria. 2. Relatives or accompanying persons of outpatients affected by neurological diseases other than ALS, and coming to the same hospital ambulatories, served as population controls. 3. Response rates not indicated. 1. 179 SALS cases. Cases have donated DNA samples to Australian MND banks, and were recruited by the MND association. More than 90% of ALS patients nationwide have been recruited into this bank. 2. No response rate was indicated. 3. Controls were normal subjects with nonneurological diseases matched on age, ethnicity, and sex. ; 1. 77 cases (57 males, 20 females, mean age=59±8 years) from 89 consecutive patients recruited from a clinic during the period 19791987. 2. Controls (80) were in-patients with various types of neurological diseases , and were matched to cases by age, sex, life-style (alcohol drinking, smoking), education, origin, and cultural background over same period 1. Cases were diagnosed by neurologists from 1978 to 1979. 2. Of the 145 patients who received the study questionnaires, 105 replies were obtained. 3.Cases included 6 fALS patients. 4. Controls were not described. 1. All individuals included in the Swedish census in 1980 who were economically active in 1. 66 cases and 66 controls. 2. 31 females. 3. There were more married ALS cases (45) than controls (30). 4. A lower level of education was seen among ALS cases (22/66 cases with high school or less education), as compared to controls (3/63). 3. Lead and mercury(unexposed/exposed): cases=51/29, controls=77/11 OR=3.98(3.20-4.76) p=0.001. Exposure to lead: 1. Occupational and residential exposure information was obtained via a mail-in questionnaire. Binary variables for specific risk factors (including lead) were created based job titles. 2. The most frequent metal exposures were welding fumes (11/8 cases/controls), lead ( 9/6), nickel (3/5), mercury (4/2), and chromates (1/3). 14 1. Cases, 77; 43, males, 34 females, 44 spinal, 32 bulbar. Age=65±9.3 years, range 42-83years; onset age=62.4 (60.164.7) years, age at diagnosis= 63.7(61.466.1) years. 2. Controls, 185, male, 69, female, 116, different from cases. Average age=57.5±13.0 years, with a range of 28-84 years. Exposure to heavy metals: 1. History of occupational exposure to heavy metals and metal fumes was identified by interview using a questionnaire based on standardized criteria in occupational epidemiology. 2. Exposed to metals or metal fumes, spinal + bulbar controls=7/178, cases=9/68, OR=1.83(0.82-4.14). 3. Exposed to metals or metal fumes, spinal, controls=7/178, cases=8/36, OR=2.94(1.20-7.21). 7 1. Cases: 125 males, 54 females, mean age=60 years (SD=10 years). 2. Controls: 125 males, 54 females,, mean age=61 years (SD=10 years). 141controls were unrelated to patients, and 38 controls are related ALS patients. Among related controls, 96 controls were spouses, 35 controls lived in the community controls, and 10 were acquaintances. No information on race was available. . Exposure to heavy metals: 1. Occupational exposure to metals was ascertained using a selfreported questionnaire, which included a question specifically about exposure to metals. 1. Both sexes combined (exposed/unexposed): cases=55/123, controls=43/136, OR=1.41(0.89-2.25). 2. Men (exposed/unexposed): cases=51/74, controls=41/84, OR=1.41(0.86-2.36). 3. Women (exposed/unexposed): cases=4/49, controls=2/52, OR=2.12(0.43-10.45). Exposure to heavy metals and hard labor: Information on occupational exposure to heavy metals was collected using a questionnaire administered via personal interview, including a question about continuous contact with specific metals within 5 years prior to disease onset. 1. Participated in hard labour: cases=54, controls=39, RR=2.4, Chi-square=6.4, P<0.05. 2. Exposed to heavy metal: cases= 7, controls=4, RR=1.8; Chisquare=1.0. Exposure to heavy metals: 1. Information of exposure to metals was obtained via a mail-in questionnaire, with a question on an employment history. 2. Of the 105 ALS cases, 26 reported exposure to one or more heavy metals; 8 of 164 were exposed. 10 1. The average age at onset was 55.6 years, ranging from 19 to 83 years. 2. There were 47 women and 58 men. 1. The age range at the start of follow-up was 16 to 98 years, with a median of 43 years. 3 Exposure to heavy metals: 1. Occupational information was identified from the 1970 and 1980 censuses; welding was considered to be an occupation 6 5 N/A (14) Gunnarsson, 1992, Sweden (15) Casecontrol Malek, 2013, USA (16) Casecontrol Park, 2005, USA (17) Cohort Study 1970 or in 1980, and who were alive on January 1, 1981, for a total of 4,812,646 subjects (2,649,300 men and 2,163,346 women). Subjects were followed from January 1, 1981 until December 31 1995 or until death, whichever came first. 1. Cases 45-79 years of age were frequency matched to a random sample of 500 population controls within the same age range. 2. A total of 112 patients diagnosed with MND in the age range 45-79 was identified in the study area, which included 1-2 million inhabitants. 3. The response rate in different counties ranged from 61-81% for controls, and from 74100% for cases. 1. SALS patients were recruited from 3 major neurology clinics, (2 in Pittsburgh and 1 in Philadelphia), , between December 2008 and July 2010. 2. Patients were diagnosed using EI Escorial. 3. fALS cases and ALS cases with other neurological conditions present were excluded. 4. Cases and controls were required to speak English. Controls were selected from the corresponding geographic region (Western Pennsylvania or the Greater Philadelphia area) and matched to cases on a 1:1 basis by age of onset/first ALS symptoms (±5 years), sex, and race. 5. Of the 106 patients contacted, 78 participated, for a response rate of 73.6%; however, only 66 cases were included in the analysis due to lack of corresponding controls. 1. Death certificate information for all deaths from 22 participating states in the years 1992– 1998 was obtained using the National Occupational Mortality Surveillance System, providing underlying and contributing causes of death. 2. Controls were all decedents with no mention of neurologic disease, degenerative or otherwise, and excluding: accidental causes, malignant neoplasms of the brain (ICD 191), other senile and presenile organic psychotic conditions (ICD 290), diseases of the nervous 2. The number of male ALS cases was 1,411 with an average age of 70 years; the number of females cases was 554 with average age of 69 years. involving exposure to heavy metals. 2. Among welders, there were 24 cases, with an estimated RR=1.6 (1.1–2.4). 1. Completed questionnaires were received from 92 MND cases (58 men and 34 women) and 372 controls (189 men and 183 women), corresponding to a response rate of 85% among the cases (men 83% and women 89%) and 75% among the controls (men 74% and woman 75%). 2. Among the controls, the proportion of respondents was proportionately lower than among those working in agriculture or forestry (63%), but among the cases, this proportion was equal to other occupations. The majority of cases and controls were male (68.2%), Caucasian/White (98.5%) and from Western Pennsylvania (86.4%). Cases (mean ± SD: 57.1 ± 13.2 years) were slightly older than controls (56.4 ± 13.5 years), although this difference did not achieve statistical significance (p = 0.07). Exposure to heavy metals: 1. Occupational information was obtained by mail-in questionnaire using welding as a proxy for lead exposure to lead vapour. 2. The ORs for lead and welding were 2.8(0.7-9.2) and 3.7 (1.113.0), respectively. N/A Exposure to heavy metals: 1. Exposure information in for occupations held for at least two years after 19 years of age was obtained by personal interview, according to the 1980 US Census industrial and occupational classification system. 2. Regarding heavy metals, there were 19/66 cases/controls exposure, leading to an OR=0.89 (0.84-4.24). 2. After controlling for smoking (ever vs. never) and education (high school or less vs. more than high school), cases reported significantly greater occupational exposure to metals (OR =3.65; 95% CI: 1.15, 11.60) and pesticides (OR = 6.50; 95% CI: 1.78, 23.77) than controls. 3. Occupational exposure to electrical or electronic equipment, machinery or electromagnetic fields again appeared to be protective against ALS (OR =0.28; 95% CI: 0.11, 0.69). 4. Additional individual risk factors were also considered, but the data was not shown because the sample size was too small. N/A The number of decedent participants is given below. Exposure to heavy metals: 1. Information on occupational exposure to heavy metals was obtained from the National Occupational Mortality Surveillance System. Occupations were specified as mutually exclusive subsets of Bureau of the Census occupation codes. 2. There were 44,545 deaths in welding occupations, including 70 ALS cases. 3. The standardized mortality ratio was 0.66 (0.49-0.88) for age >65 years; 0.89(0.53-1.47) for age <-65 years, adjusted for age, race, gender, region and socioeconomic status. N/A Group White men White women Non-white men Deaths due to ALS 3,851 2,152 203 4 Total deaths 1,479,921 803,110 203,862 Qureshi, 2006, USA (18) Case control system and sense organs (ICD 320–389), and neoplasms of the lymphatic and hematopoietic tissues (ICD 200–208), due to suspect associations with solvents or electromagnetic fields (EMFs). 1. 95 subjects with ALS and 106 healthy control subjects between April 1998 and August 2002. 2. Cases were identified from clinic. Controls were non-blood relatives (spouses), friends, or unrelated subjects. 3. At recruitment controls were matched to the ALS subjects by gender and age. Non-white women Total Fang , 2010, USA, (20) Casecontrol Casecontrol 1. All study participants (cases and controls) were recruited between 1993 and 1996. 2. ALS cases were recruited from two major referral centers in New England. 3. Cases were required to live in New England for at least 50% of the year, to be mentally competent, and to speak English. 71% of eligible cases participated in the study (n = 111). About 85% of the cases were enrolled within 1 year of diagnosis and the remainder within 2 years. 4. Population controls were identified through random telephone screening, had no neurological disease, and were sex, age (30– 55, 56–65, and 66–80 years) and region matched to cases. 354 eligible controls were contacted, 270 (76%) were enrolled, 256 completed the entire questionnaire. 1. Included a subset of registry cases comprising MND cases who donated blood between January 23, 2007 and September 30, 2007; 208 cases were eligible, of whom 200 were enrolled, including 163 ALS cases (including 12 possible cases), 30 progressive muscular atrophy cases, and 7 primary lateral sclerosis cases. [Note: most studies only use definite and probable cases.] 2. Between May 2007 and May 2008, contacted 359 controls already enrolled in this study for additional informed consent and blood sample collection. A total of 252 controls consented to participate in this study, of which 229 ultimately donated a blood sample. 127,453 6,347 2,614,346 Characteristics of the study subjects are summarized below. Group Male Caucasian Mean age Year± SD Fang, 2009, USA (19) 141 Cases 60 (63.2%) 91 (95.8%) 54.4 ±13.1 Controls 58 (54.7%) 102 (96.2%) 52.5 ±14.9 1. The median age at diagnosis for cases was 60 years (range 30–79 years) and the median age 2 years before interview for controls was 59 (29–78)years. Diagnosis was made an average of 14.3 months after the onset of symptoms. 2. Males: cases, 66 (60.6%); controls, 156 (61.7%). Females: cases, 43 (39.4%); controls, 97 (38.3%) 3. 27 (25%) of the cases had bulbar onset and 82 (75%) had trunk or limb onset. Exposure to lead-heavy metals: 1. Each ALS and control subject completed a comprehensive risk factor questionnaire at enrolment. Subjects answered questions about risk factors for ALS, disease presentation (ALS subjects only), and family and medication histories. 2. Of the 95 subjects in the ALS group, the exposure most commonly reported was exposure to pesticides (n=18), followed by lead (15), industrial solvents (5), mercury (5) and miscellaneous agents (number is not specified). . N/A Exposure to lead: As in Kamel et al. (2006) above, occupational history information was obtained via interview. Duration of occupational exposure to lead was categorized into four levels (0, 1–399,400–1,999, and 2,000 days of exposure). The number of cases and controls exposed to lead at least 10 times and accumulated for one day is summarized below. N/A Group Overall Smokers Non-smokers Cases 55 33 22 Controls 35 25 10 OR 1.9(1.1-3.4) 1.9(0.9-3.8) 2.8(1.0-7.8) Cases (exposed/unexposed)= 35/84, Controls(exposed/unexposed)=55/201. 1. Participants were recruited from 2003–2007. 2. 184 cases and 194 controls among US veterans; most are male (98% of cases, and 94% of controls) and white (94% of cases, and 98% of controls). 5 Exposure to lead: Lead concentrations in blood samples were determined by inductively coupled plasma mass spectrometry. 1. After adjustment for age, a unit increment of log2-transformed lead (equivalent to a doubling of blood lead) was associated with a 2.6-fold higher odds of ALS (95% confidence interval: 1.9, 3.7). Adjustment for smoking (ever/never) in addition to age did not materially change the results. 2.A dose response for the lead-ALS association was also seen when blood lead was categorized in tertiles; after adjustment for age and type 1 collagen, the odds ratio for the highest compared with the lowest tertile was 2.1 (95% confidence interval: 1.1, 3.8; P for trend= 0.008). N/A Kamel, 2005, USA (21) Casecontrol 3. Response rate: cases, 200/208; controls, 229/252. 1. All study participants (cases and controls) were recruited between 1993 and 1996. 2. ALS cases were recruited from two major referral centers in New England. 3. Cases were required to live in New England for at least 50% of the year, to be mentally competent, and to speak English. 71% of eligible cases participated in the study (n = 111). About 85% of the cases were enrolled within 1 year after diagnosis and the remainder within 2 years. 4. Population controls were identified through random telephone screening, no neurological disease, sex, age (30–55, 56–65, and 66–80 years) and region matched. 354 eligible controls were contacted, 270 (76%) were enrolled, 256 completed the entire questionnaire. 1. The median age at diagnosis for cases was 60 years (range 30–79 years; the median age at 2 years before interview for controls was 59 years (range 29–78 years). Diagnosis was made 14.3 months after the onset of symptoms on average. 2. Males: cases, 66 (60.6%); controls, 156 (61.7%). Females: cases, 43 (39.4%); controls, 97 (38.3%) 3. 27 (25%) of the cases had bulbar onset and 82 (75%) had trunk or limb onset. Exposure to lead 1. Ever exposure to lead (unexposed/exposed): cases=67/35, controls= 193/53; OR=1.9(1.4-2.6). 2. Gradient in lead exposure across tertiles (number, p trend=0.02): Days exposed to lead No. of ALS cases N/A No. of control s OR 0 67 193 1 1-399 8 17 1.6 (0.6-3.9) 400-1999 11 17 1.9 (0.8-4.3) 2000+ 16 20 2.3 (1.1-4.9) 3.Blood lead levels (µg/dl): cases=5.2±0.3; controls=3.4±0.4 (p<0.05). References 1. Armon C, Kurland LT, Daube JR, et al. Epidemiologic correlates of sporadic amyotrophic lateral sclerosis. Neurology. 1991;41(7):1077-84. 2. Campbell AM, Williams ER, Barltrop D. Motor neurone disease and exposure to lead. J Neurol Neurosurg Psychiatry. 1970;33(6):877-85. 3. Chancellor AM, Slattery JM, Fraser H, et al. Risk factors for motor neuron disease: a case-control study based on patients from the Scottish Motor Neuron Disease Register. J Neurol Neurosurg Psychiatry. 1993;56(11):1200-6. 4. Deapen DM, Henderson BE. A case-control study of amyotrophic lateral sclerosis. Am J Epidemiol. 1986;123(5):790-9. 5. Gresham LS, Molgaard CA, Golbeck AL, et al. Amyotrophic lateral sclerosis and occupational heavy metal exposure: a case-control study. Neuroepidemiology. 1986;5(1):29-38. 6. Kamel F, Umbach D, Munsat T, et al. Lead exposure and amyotrophic lateral sclerosis. Epidemiology. 2002;13(3):311-9. 7. McGuire V, Longstreth WT,Jr, Nelson LM, et al. Occupational exposures and amyotrophic lateral sclerosis. A population-based case-control study. Am J Epidemiol. 1997;145(12):1076-88. 8. Pierce-Ruhland R, Patten BM. Repeat study of antecedent events in motor neuron disease. Ann Clin Res. 1981;13(2):102-7. 9. Yu Y, Su FC, Callaghan BC, et al. Environmental Risk Factors and Amyotrophic Lateral Sclerosis (ALS): A Case-Control Study of ALS in Michigan. PLoS One. 2014;9(6):e101186. (doi: 10.1371/journal.pone.0101186; 10.1371/journal.pone.0101186). 10. Binazzi A, Belli S, Uccelli R, et al. An exploratory case-control study on spinal and bulbar forms of amyotrophic lateral sclerosis in the province of Rome. Amyotroph Lateral Scler. 2009;10(56):361-9. (doi: 10.3109/17482960802382313). 11. Morahan JM, Pamphlett R. Amyotrophic lateral sclerosis and exposure to environmental toxins: an Australian case-control study. Neuroepidemiology. 2006;27(3):130-5. (doi: 10.1159/000095552). 12. Provinciali L, Giovagnoli AR. Antecedent events in amyotrophic lateral sclerosis: do they influence clinical onset and progression? Neuroepidemiology. 1990;9(5):255-62. 13. Roelofs-Iverson RA, Mulder DW, Elveback LR, et al. ALS and heavy metals: a pilot case-control study. Neurology. 1984;34(3):393-5. 14. Feychting M, Jonsson F, Pedersen NL, et al. Occupational magnetic field exposure and neurodegenerative disease. Epidemiology. 2003;14(4):413,9; discussion 427-8. (doi: 10.1097/01.EDE.0000071409.23291.7b). 15. Gunnarsson LG, Bodin L, Soderfeldt B, et al. A case-control study of motor neurone disease: its relation to heritability, and occupational exposures, particularly to solvents. Br J Ind Med. 1992;49(11):791-8. 16. Malek AM, Barchowsky A, Bowser R, et al. Environmental and Occupational Risk Factors for Amyotrophic Lateral Sclerosis: A Case-Control Study. Neurodegener Dis. 2013. (doi: 10.1159/000355344). 17. Park R, Schulte P, Bowman J, et al. Potential occupational risks for neurodegenerative diseases. Am J Ind Med. 2005;48(1):63-77. 18. Qureshi MM, Hayden D, Urbinelli L, et al. Analysis of factors that modify susceptibility and rate of progression in amyotrophic lateral sclerosis (ALS). Amyotroph Lateral Scler. 2006;7(3):173-82. (doi: 10.1080/14660820600640596). 19. Fang F, Quinlan P, Ye W, et al. Workplace exposures and the risk of amyotrophic lateral sclerosis. Environ Health Perspect. 2009;117(9):1387-92. (doi: 10.1289/ehp.0900580). 6 20. Fang F, Kwee LC, Allen KD, et al. Association between blood lead and the risk of amyotrophic lateral sclerosis. Am J Epidemiol. 2010;171(10):1126-33. (doi: 10.1093/aje/kwq063; 10.1093/aje/kwq063). 21. Kamel F, Umbach DM, Hu H, et al. Lead exposure as a risk factor for amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2(3-4):195-201. (doi: 10.1159/000089625). 7






