Exp. 9: Measurement of Nitrates in water
advertisement
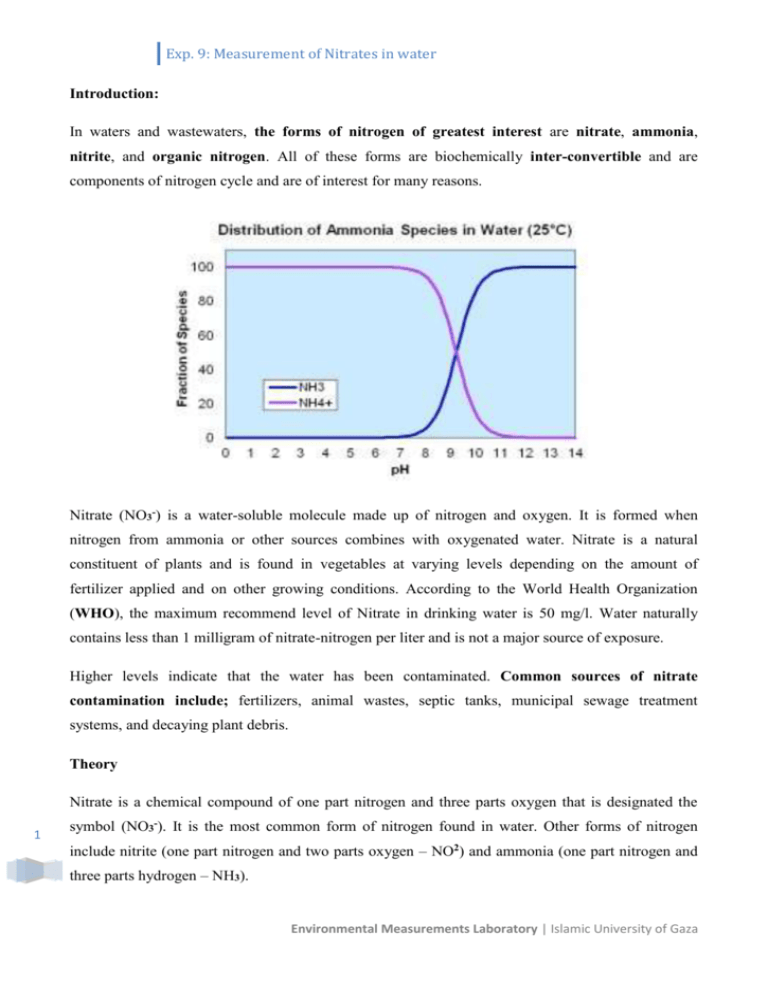
Exp. 9: Measurement of Nitrates in water Introduction: In waters and wastewaters, the forms of nitrogen of greatest interest are nitrate, ammonia, nitrite, and organic nitrogen. All of these forms are biochemically inter-convertible and are components of nitrogen cycle and are of interest for many reasons. Nitrate (NO3-) is a water-soluble molecule made up of nitrogen and oxygen. It is formed when nitrogen from ammonia or other sources combines with oxygenated water. Nitrate is a natural constituent of plants and is found in vegetables at varying levels depending on the amount of fertilizer applied and on other growing conditions. According to the World Health Organization (WHO), the maximum recommend level of Nitrate in drinking water is 50 mg/l. Water naturally contains less than 1 milligram of nitrate-nitrogen per liter and is not a major source of exposure. Higher levels indicate that the water has been contaminated. Common sources of nitrate contamination include; fertilizers, animal wastes, septic tanks, municipal sewage treatment systems, and decaying plant debris. Theory Nitrate is a chemical compound of one part nitrogen and three parts oxygen that is designated the 1 symbol (NO3-). It is the most common form of nitrogen found in water. Other forms of nitrogen include nitrite (one part nitrogen and two parts oxygen – NO2) and ammonia (one part nitrogen and three parts hydrogen – NH3). Environmental Measurements Laboratory | Islamic University of Gaza Exp. 9: Measurement of Nitrates in water In water, nitrate has no taste or scent and can only be detected through a chemical test. The Maximum Acceptable Concentration (MAC) for nitrate in drinking water in British Columbia is 45 milligrams per litre (mg/L). For laboratory tests reported as nitrate-nitrogen (NO3-N, the amount of nitrogen present in nitrate) the MAC is 10 mg/L while the maximum acceptable concentration in Palestinian standard is 50 mg/L. Generally the ground water has high nitrate concentration because of the percolating sewage, industrial waste, chemical fertilizers, leaches from solid waste landfills, septic tank effluents etc. Whatever may be the reason the high concentration of nitrate is harmful to human beings, particularly for infants. The low acidity in the infants’ intestine permits the growth of nitrate reducing bacteria that converts the nitrate to nitrite that is then absorbed in the blood stream. The nitrite has a great affinity for haemoglobin than the oxygen and it replaces oxygen in the blood. The deficiency of oxygen causes suffocation. The colour of the skin of the infants becomes blue so it is termed as blue baby disease. The medical name is ‘mathemoglobinemia’. This disease is a fatal disease and it takes place when the concentration of nitrates is more than 50 ppm. So it is important to find the amount of nitrate in drinking water though it is a difficult task and requires spectrophotometer also. Nitrate can be removed from drinking water by; ion exchange, reverse osmosis, electro dialysis. Health Effects Infants who are fed water or formula made with water that is high in nitrate can develop a condition that doctors call methemoglobinemia. The condition is also called "blue baby syndrome" because the skin appears blue-gray or lavender in colour. This colour change is caused by a lack of oxygen in the blood. All infants under six months of age are at risk of nitrate poisoning. Some babies may be more sensitive than others. Infants suffering from "blue baby syndrome" need immediate medical care because the condition can lead to coma and death if it is not treated promptly. Apparatus 2 Spectrophotometer, for use at 220 nm and 275 nm with matched silica cells. Environmental Measurements Laboratory | Islamic University of Gaza Exp. 9: Measurement of Nitrates in water Reagents 1) Standard nitrate solution ( 5, 10, 15,20 and 25 mg/L). 2) Nitrate-free water: Procedure 1) Prepare standard (NO3-) nitrate solution: 0, 5, 10, 15, 20 . . . 35.0 mL, as shown; Stock solution ( 1000 mg/l ) → 5 ml/100 ml dH2O (50mg/L) From 50mg/L 20ml/50dH2O 20 mg/L 15ml/50dH2O 15 mg/L 10ml/50dH2O 10 mg/L 5ml/50dH2O 5 mg/L 2.5ml/50dH2O 2.5 mg/L 2) Spectrophotometric measurement: Read absorbance or transmittance against redistilled water set at zero absorbance. 3) Use a wavelength of 220 nm to obtain absorbance of standard nitrate solution. 4) Draw the relationship between concentration and absorbance. 5) Calculate the factor m (slope of line). 6) Calculate the concentration of nitrate from the standard curve or from equation; Conc. (Y-axis) = Slope * Absorbance (X-axis) Calculations From plotting the calibration linear curve, find the concentrations of the water samples; 3 Concentration mg/l absorption 2.5 0.19 5 0.35 10 0.73 15 1.11 20 1.50 3 unknown samples Environmental Measurements Laboratory | Islamic University of Gaza Exp. 9: Measurement of Nitrates in water Wael sample (??) 0.27 Khaled sample (??) 0.86 Hatem sample (??) 1.43 4 Environmental Measurements Laboratory | Islamic University of Gaza







