Example of LASD lab report
advertisement

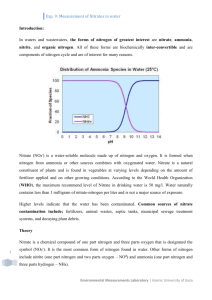
STUDENT NAME 2/8/13 Quantitative Water Analysis of Muskingum River, Ohio AP Environmental Science Purpose: In this lab we used Lab-Aids kits to determine if the water we got from a river had various pollutants in it. Materials: Empty bottle of water, filled with river or creek water Lab-Aids #19 A Qualitative Introduction to Water Pollution Kit (see attached lab sheet) Mr. Wagner's nitrate nitrogen test solution #1 Calibrated test tube Plastic spatula Chemplate Pipette Test tube cap Procedure: See attached lab sheet. To get my sample I went to Putnam Landing, Zanesville, Ohio on 2/2/13 and filled my bottle with river water from the Muskingum River. On test 8- Nitrate nitrogen, instead of using the Nitrate Test Solution #1, we used the solution Mr. Wagner created that we think is the same as the Nitrate Test Solution #1. Data: TEST NAME Ammonia Nitrogen COLOR OBSERVED Light Yellow (+) OR (-) + pH Chlorine Chromium Copper Cyanide Iron Nitrate Nitrogen Phosphorous Silica Sulfide Green Same as creek water Same as creek water Same as creek water Same as creek water Same as creek water Cloudy pink Very dark, navy blue Medium blue Same as creek water pH- 8 + + + - Discussion: The purpose of this lab was to test the sample of river water for various pollutants using the Lab-Aid kits. The sample of water I took from the Muskingum tested positive for Ammonia Nitrogen. Some sources of this chemical are fertilizers, and human and animal waste. The Muskingum River is in an area with a lot of agriculture, so fertilizer run-off might have gotten into the river. An excess of this can be toxic to aquatic life. The river water I sampled also tested positive for Nitrate Nitrogen, turning a cloudy pink. Nitrogen can get into the water from fertilizer run-off, sewage, animal waste, and industrial and municipal waste water. When there is an excess amount of nitrate nitrogen in the water eutrophication can happen. This causes an increased photosynthetic productivity and algae blooms. When the algae dies the microorganisms that decompose it use up all of the dissolved oxygen. Then there is less dissolved oxygen that other aquatic animals need to survive. The Phosphorus test I did on the river water also tested positive. This also comes from runoff from fertilizers, manure and animal waste. Like nitrate nitrogen, this chemical, in above-average concentration, can cause eutrophication and lack of dissolved oxygen. The Muskingum River sample also tested positive for silica. This chemical can get into rivers from weathering of rocks and soil. I researched and found that a river's pH is normally 6.9. That means the Muskingum River is slightly basic. River water can become more alkaline by dissolving minerals like rocks and soil. That makes sense because there was silica in the water and that also comes from weathering rocks and soil. One source of possible error would be that some of the materials were washed with school water which put other chemicals in the tests. Also I used an empty bottle of water to get my sample. The bottled water had chemicals like magnesium sulfate and potassium chloride in it. I might not have completely cleaned out the bottle when I got the sample and that could have affected the test results. One question that I was thinking was, Are there poultry farms that I could not find on Google Earth in the area where I got the sample? I was wondering this because there was a lot of phosphorus in the water I tested and that often comes from chicken waste.