Primary culture of astrocytes (detailed protocol)

Supplementary Information: Protocol for primary culture of mice microglia.
Primary mouse cultures of cortical mixed glial cells were prepared from 1-3 day-old neonatal mice, as previously described (Giulian, D., T. J. Baker. 1986). Briefly, cerebral cortices were dissected, carefully stripped of their meninges, and digested with 0.25% trypsin for 30 min at 37°C. Trypsinization was stopped by adding an equal volume of culture medium (DMEM/F-12; Invitrogen) with 14% FBS and 0.02% DNase I. The solution was pelleted, resuspended in culture medium, and brought to a single cell suspension by repeated pipetting following by passage through a 90-μm pore mesh.
Cells were seeded at density of 125000 cells/ml. Cells were maintained at 37°C in humidified 5% CO2/95% air. Medium was replaced every 4-5 days, and cultures were used between 14-20 days in vitro. Then to isolate the murine microglia, the procedure of
Saura et al (2003 was followed. Briefly, confluent mixed glial cultures were subjected to mild trypsinization (0.05%) in the presence of 0.25 mM EDTA and 0.5 mM Ca2+.
This results in the detachment of astroglial cells and leaves a population of firmly attached cells identified as 95-98% microglia, as determined by immunofluorescence using anti-CD11b, anti-GFAP, and anti-mitogen-activated protein-2 (Fernandez-Lizarbe et al., 2009). After 24 h of trypsinization, cells were rinsed and incubated in serum-free medium with N-2 Supplement (Invitrogen) for 18 h. Then microglial cells were incubated with the different treatments
Giulian, D. and Baker, T.J. (1986) Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci 6, 2163-2178.
Saura, J., J. M. Tusell, and J. Serratosa. 2003. High-yield isolation of murine microglia by mild trypsinization. Glia 44: 183–189.
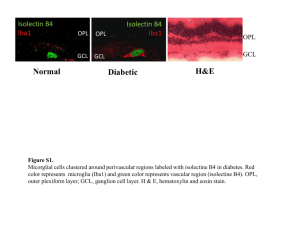
Supplementary Figure 1: Purity of the primary culture of the cortical microglia.
Microglia purity was assessed by immunofluorescence. Thus the microglia cells in culture were incubated for 10 min in PBS that contained 0.1% Triton X-100, rinsed off with PBS (1×) and blocked for 1 h/RT with 5% normal goat serum in TBS/T (0.1%).
After blocking, sections were incubated with mouse monoclonal anti-GFAP (1/250,
Sigma Aldrich) and anti-Iba-1 (2µg/mL, Wako Chemicals), followed by Alexa Fluor
488 and 647 (1/500, Molecular Probes, respectively). Samples were counterstained with
DAPI (1 µg/mL) as a nuclei marker. Sections were mounted onto glass slides with fluorescent mounting medium (Dako North America). For the negative controls, sections were incubated in parallel with either the antibody diluent alone or the specific
IgGs isotype control antibodies to block the non specific staining. The percentage (%) of GFAP and Iba-1 positive cells was evaluated. Approximately 2000 cells (nuclei) were counted. The cultured microglia were found to be [97.43 ± 0.52] % of the Iba-1 positive cells (A) and [3.5 ± 0.52] % of the GFAP positive cells (B). A representative microglia cell morphology in cell culture is shown (C-D)Scale bar, 10
m.