22/11/2012
advertisement
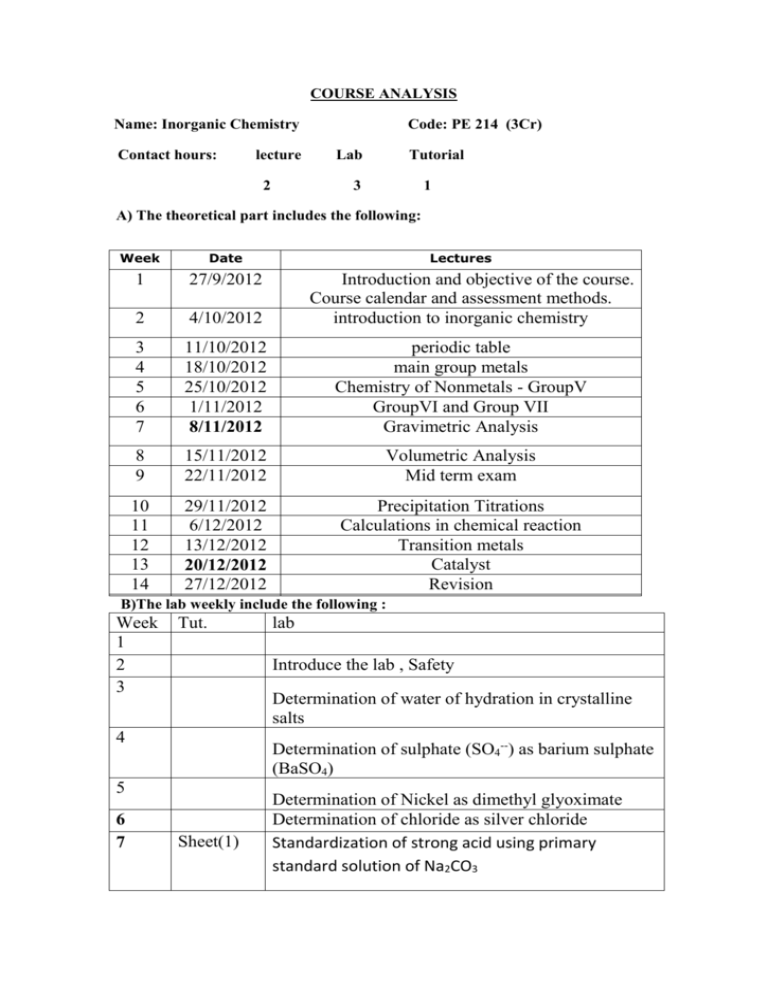
COURSE ANALYSIS Name: Inorganic Chemistry Contact hours: lecture 2 Code: PE 214 (3Cr) Lab Tutorial 3 1 A) The theoretical part includes the following: Week Date Lectures 1 27/9/2012 2 4/10/2012 3 4 5 6 7 11/10/2012 18/10/2012 25/10/2012 1/11/2012 8/11/2012 periodic table main group metals Chemistry of Nonmetals - GroupV GroupVI and Group VII Gravimetric Analysis 8 9 15/11/2012 22/11/2012 Volumetric Analysis Mid term exam 10 11 12 13 14 29/11/2012 6/12/2012 13/12/2012 20/12/2012 27/12/2012 Precipitation Titrations Calculations in chemical reaction Transition metals Catalyst Revision Introduction and objective of the course. Course calendar and assessment methods. introduction to inorganic chemistry B)The lab weekly include the following : Week 1 2 3 Tut. Introduce the lab , Safety Determination of water of hydration in crystalline salts 4 Determination of sulphate (SO4--) as barium sulphate (BaSO4) 5 6 7 lab Sheet(1) Determination of Nickel as dimethyl glyoximate Determination of chloride as silver chloride Standardization of strong acid using primary standard solution of Na2CO3 8 Sheet (2) 9 Standardization of strong acid using primary standard solution of Na2CO3 Mid term exam 10 Sheet (3) 11 Sheet(4) 12 13 14 15 Determination of the strength of sulphuric acid using standard NaOH Determination of ammonia in ammonium chloride Neutralization of a mixture of carbonate (CO--), bicarbonate (HCO3-), and hydroxide (OH-) Determination of Fe++ ion as FeSO4 Determination of oxalate Final lab exam Assessment: * midterm exam --------------------------------20 %( 9thweek exam) * Quizzes ------------------------------------- ----10% * Attendance and lab work --- -- -------------- 10% * Exam lab ----------------------------------10% * Final exam ------------------------50% D) Aim of the course & intellectual skills The students will know strong background in chemistry and its practical applications in industry. Know the trends of periodic table Know properties of metals, know properties of nonmetals Calculate molecular weight of the compound, Calulate number of moles , Balance molecular equation Calculate molarity of solution, Calculate normality of solution Know the oxidation number, Know the reducing agents and reducing agents Calculate the solubilty product in solution, Text book: Vogel’s Quantitative Chemical Analysis, by: J. Mendham, R.C. Denney, J. D. Barners, M.J.K. Thomas, 1999. Office Hours:Monday 11:30-2:30, Tuesday : 8:30-10:30, Thursday : 9:30-11:30











