Activity 4-Evaporation Is Cool
advertisement

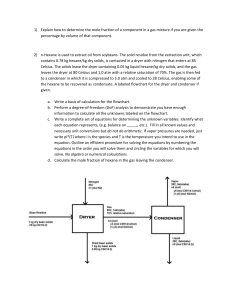
Physical Science Institute Summer 2013 Evaporation Is Cool MATERIALS (1) bottle w/ hexane (1) bottle, w/ purified water (1) bottle, w/ ethyl alcohol (3) test tubes, 20x150mm (1) test tube rack (1) stainless temperature probe (1) LabQuest2 data collection device (3) small orthodontic rubber bands (1) roll of labeling tape (3) 1”x ½” pieces cut from a sheet of 8” x 8” chromatography paper PROCEDURE-Part 1 Predict Which liquid below will have the highest rate of evaporation? Water : H2O ethyl alcohol: C2H5OH hexane: C6H14 Test your prediction. Place an equal amount of ethyl alcohol, hexane, and water on three separate cotton balls. Wipe the cotton balls on the desk at the same time. Observe the relative rates of evaporation. Questions 2. Which liquid had the greatest rate of evaporation? 3. Which liquid had the slowest rate of evaporation? 4. Which of the three liquids has the strongest attraction between the molecules, i.e., greatest intermolecular forces? Support your answer using your observations about rates of evaporation. . PROCEDURE-Part 2 Predict Which liquid (water, ethyl alcohol, or hexane) will produce the greatest temperature change as it evaporates? Test your prediction. 1. Place water in a test tube to about ¼ full. In separate test tubes, do the same with ethyl alcohol and again with hexane. 2. Turn the LabQuest2 on. In the LabQuest App, tap the Meter tab and set up the data collection parameters: a. Mode: Time Based b. Interval: 1 s/sample c. Duration 300 seconds 3. Wrap the probe with a piece of filter paper supplied by your instructor. Secure the filter paper by a small rubber band as shown in Fig 1. Roll the filter paper around the probe tip in the shape of a cylinder. Hint: First slip the rubber band on the probe, wrap the paper around the probe and then slip the rubber band over the paper. The paper should be even with the probe end. 4. Stand the probe in the test tube containing hexane. 1 Physical Science Institute Summer 2013 5. Prepare 2 pieces of masking tape, each about 10 cm long, to be used to tape the probes in position during step 7 6. After the probe has been in the test tube containing hexane for at least 30 seconds, select to begin collecting temperature data. Then remove the probe from the hexane and tape it so the probe tip extends 5 cm over the edge of the table top as shown in Figure 1. 7. Data collection will stop after 4 minutes. Examine the graph of temperature vs. time. Based on your data, determine the maximum and minimum temperatures for each liquid. Record these temperatures. 8. Remove the filter paper from the probe and save the rubber band. Get a new piece of filter paper and repeat steps 27 with ethyl alcohol and then water. Liquid DATA Min. Temperature (oC) Max. Temperature (oC) hexane ethyl alcohol water CALCULATIONS AND RESULTS 1. For each liquid, subtract the minimum temperature from the maximum temperature to determine the temperature change (t) during evaporation. Liquid t (oC) hexane ethyl alcohol water 2. How did your data compare to your prediction? 3. What is the relationship (inverse or direct) between the amount the temperature changed as the liquid evaporated and the strength of molecular attractions? 2