mmr vaccine and autism - Association of American Colleges
advertisement

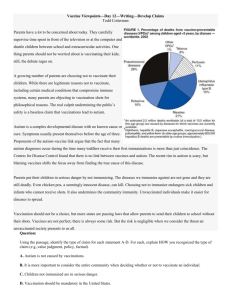
STUDENT CASE STUDY, PART TWO B: CASE CONTROL STUDY― SINGER-FREEMAN MMR VACCINE AND AUTISM: SCIENTIFIC INQUIRY, ETHICS, AND EVIDENCEBASED PROBLEM SOLVING CASE STUDY FOR THE AACU STIRS PROGRAM Karen Singer-Freeman, Associate Professor of Psychology, Purchase College, State University of New York STUDENT CASE Part Two B: Case Control Study How Can We Study the Effects of Potentially Dangerous Treatments? Methods Used in the Study of Vaccines and Autism Case Control Study In a case control study we identify individuals with a disease (cases) and find other individuals (controls) who do not have this disease but are otherwise similar to those with the disease in as many ways as possible. To increase the statistical power (the probability that a statistical test will correctly reject the null hypothesis when the null hypothesis is false) of the research design, each case is often matched with multiple controls, particularly if the number of cases is limited. Matching controls to cases on age, sex, or other known disease risk factors increases the likelihood that the controls as a group will be similar to the case in important ways. Once the cases and controls have been selected, the researcher looks back in time using surveys, medical records, or other forms of previously collected data to determine whether the cases or the controls in the study received the treatment of interest, in this case the MMR vaccine (the hypothesized trigger for autism). Researchers determine whether cases are more likely to have experienced a potential trigger for their disease than controls. If exposure to the hypothesized trigger is higher among cases than controls, it suggests that there might be an association between the trigger and control. However, a case control study will not allow us to conclude that the MMR vaccine causes autism because the cases and controls we select may differ in other important ways. Because we did not randomly assign subjects to groups, there may be other differences between the groups that cause the differences we believe to be resulting from our exposure (in this case, the MMR vaccination). Accordingly, even when an association is seen between a trigger and a disease the researcher cannot conclude that the trigger causes the disease. Figure 7 summarizes the key features of case control design. STUDENT CASE STUDY, PART TWO B: CASE CONTROL STUDY― SINGER-FREEMAN Children with Autism are identified Each child with Autism is matched with a similar child who does not have Autism History of MMR vaccination is collected from medical records for both groups of children and compared Figure 7. A Case Control Design to Examine the Association between the MMR Vaccine and Autism. A Real Case Control Study Uno, Uchiyama, Kurosawa, Aleksic, and Ozaki (2012) identified 189 individuals with autism (cases) and 224 controls who were matched by sex and birth year to the cases. Key Questions 23) Are there any other risk factors the cases and controls should have been matched for? Explain your answer. 24) If the MMR vaccine causes autism, which group should have had a higher rate of vaccination coverage (meaning a higher percentage of children in this group received the MMR vaccine)? Uno et al. 2012 found that vaccination records were not significantly different between the cases and the controls. The authors concluded that neither receipt of the MMR vaccine nor the total number of vaccines received were associated with increased risk of autism. Key Questions 25) Can you think of a reason we might not see higher vaccination rates among children with autism even if the MMR vaccine does cause autism in some children? Propose another factor that could cause children to develop autism. 26) Would it be useful to record the date of the MMR vaccine and the date of autism diagnosis for each child in the study? Explain your answer. 27) Propose another way to study the association between the MMR vaccine and the development of autism. Be sure to include the group or groups you would study, the information you would gather, and how this study would add to our understanding of vaccine safety. Once your group is done with these questions, please see your instructor for guidance on next steps.