jec12110-sup-0001-suppinfo_TableS1-S3_FigS1-S3
advertisement

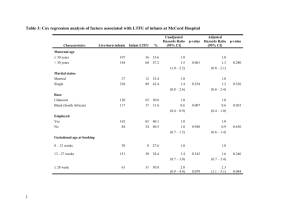
Appendix S1. Supplementary methods and results. Graphical vector analysis (GVA): To construct the GVA plots in Fig. 4A and B, we converted the individual foliar chemical content, concentration, and biomass of offspring of each taxon into values relative to Fremont, by dividing the Fremont value by the taxon value. Analogously, for Fig. 4C and D, we converted the individual foliar chemical content, concentration, and biomass of offspring that experienced maternal herbivory into values that are relative to the maternal control values by dividing the maternal control value by the maternal herbivory value within specific taxa (Haase & Rose 1995; Koricheva 1999). We constructed each of the graphs by first plotting the control point, where relative content (X-axis) and concentration (Y-axis) equals one. This point represents Fremont in Fig. 4A and B, and the maternal control in Fig. 4C and D. We then extended a diagonal line with a slope of one (i.e., relative content = relative concentration) from the graph origin to represent the control reference for individual foliar biomass; treatment vectors falling on the left or right side of the diagonal line represent decreased or increased leaf biomass, respectively (Veteli et al. 2007). Seedling traits in taxon, relative to those of Fremont (Fig. 4A,B), or seedling responses to the maternal herbivory treatment, relative to the maternal control (Fig. 4C,D), are then illustrated by plotting phytochemical content against concentration. Molecular analysis: We used FAM, NED or HEX 5′-labeled oligonucleotide primers (Integrated DNA Technologies, Inc., San Diego, CA, USA) for the genotyping. Reaction mixtures contained 20 ng of DNA, 50 ng of each SSR primer, 200 µmol/L dNTPs, 0.5 U Taq DNA polymerase/µL (Omega Bio-tek Inc., Norcross, GA, USA), 10 µmol/L Tris–HCl (pH 8.3), 50 mmol/L KCl, 2.0 mmol/L MgCl2, and 0.1 mg bovine serum albumin/mL. We used the following ‘touchdown’ amplification conditions: initial denaturation step at 95 ºC for 5 minutes, followed by 10 cycles of 95 ºC for 15 seconds, 58 ºC for 15 seconds (decreasing by 1 ºC each cycle), and 72 ºC for 30 seconds, followed by an additional 20 cycles of 95 ºC for 15 seconds, 50 ºC for 15 seconds and 72 ºC for 30 seconds, and final extension at 72 °C for 5 minutes. We diluted reaction products up to 1:20, denatured in 1 HiDi formamide containing a 600-bp LIZ standard (Applied Biosystems, Foster City, CA, USA). Finally, we visualized samples on an ABI 3730xl genetic analyzer and analyzed the data using ABI GeneMapper 4.0 (Applied Biosystems). Supplementary results and discussion, Molecular Analysis: The seven SSR genetic markers were highly polymorphic (Table S1) and resulted in a high cumulative exclusion probability of 0.9995. The majority of the offspring from the four F1 families were successfully assigned to a taxon (149 out of 165; 90.3%) based on NewHybrids assignments. One hundred and eight (65.5%) offspring appear to be first generation backcross to narrowleaf and 41 (24.8%) offspring were F2 hybrids, which resulted from matings between two F1 parents. No successful matings between F1 mothers and Fremont cottonwoods were observed in the study. Specific paternity was determined for 60% of the offspring and these displayed very similar results to the offspring taxon assignments (χ2 = 101.285; df = 1; p < 0.0001). The fairly frequent backcross events that we observed between F1 hybrids and narrowleaf, relative to the few successful matings between two F1 hybrids and no observed backcrosses between F1 hybrids and Fremont are consistent with the unidirectional introgression of genes (from Fremont to narrowleaf) that has been documented in previous studies in the cottonwood system (Keim 1989; Martinsen et al. 2001). If the genotype x maternal herbivory interactions that we found in the F1 hybrids for phytochemical defense production are due to epistasis, it is possible that favorable gene combinations for transgenerational plasticity that arise in the F1 due to recombination could introgress into narrowleaf (but not into Fremont). The likelihood of this introgression occurring is, of course, dependent on a number of factors, including the genetic architecture behind transgenerational plasticity. 2 Table S1. Simple sequence repeat (SSR) genetic markers employed in this study, including linkage group and the number of alleles amplified. Locus GCPM_25a GCPM_2900a GCPM_3681a ORPM_202b ORPM_206b ORPM_220b WPMS_15c Linkage Group VII III VIII VIII XIX IV V No. Alleles 11 13 7 5 10 5 4 a from http://www.ornl.gov/sci/ipgc/ssr_resource.htm from Tuskan et al. 2004 c Smulders et al. 2001 b 3 Table S2. A comparison of relative phenolic glycoside concentrations and condensed tannin concentrations in different developmental stages and among taxa in cottonwood. Developmental stage Seedling Juvenile ramet Adult juvenile developmental zone Adult mature developmental zone Phenolic glycoside foliar concentration High High Moderate/High Low Condensed tannin foliar concentration Very low Low Low Rank order of taxa References NL > F1 > Fre NL > F1 * NL > F1 > Fre or F1 > NL > Fre Rehill et al., 2006; F1 > NL >/= Fre Holeski et al., in review (current ms) Holeski et al., 2012; High 4 Table S3. Results of GLM ANOVAs for total phenolic glycoside content (A and B) and concentration (C and D) in seedlings with F1 hybrid mothers. Factors in bold print had a significant effect on the particular variable of interest. Factors in bold, grey print have a marginally significant effect on the particular variable of interest. We could not calculate a maternal genotype x paternal taxon x maternal herbivory interaction, as low representation of the F1 paternal taxon led to a highly unbalanced design. Parts B and D eliminate the possible effect of that interaction by considering only seedlings with narrowleaf fathers; these represent the majority (more than 2/3) of the seedlings with F1 mothers. Response variable A. Total phenolic glycoside content B. Total phenolic glycoside content C. Total phenolic glycoside concentration D. Total phenolic glycoside concentration Factor Foliar dry leaf weight Paternal taxon Maternal genotype Maternal herbivory Maternal genotype x maternal herbivory Foliar dry leaf weight Maternal genotype Maternal herbivory Maternal genotype x maternal herbivory Paternal taxon Maternal genotype Maternal herbivory Maternal genotype x maternal herbivory Maternal genotype Maternal herbivory Maternal genotype x maternal herbivory 5 F statistic 389.27 10.98 0.24 0.26 1.37 df 1,135 1,135 3,133 1,135 3,135 pvalue <0.001 0.001 0.867 0.640 0.254 332.63 0.16 0.02 2.36 1,95 3,95 1,95 3,95 <0.001 0.918 0.891 0.076 6.97 1.09 0.17 3.90 1,137 3,137 1,137 3,137 0.009 0.356 0.679 0.010 0.17 0.09 5.01 3,97 1,97 3,97 0.908 0.779 0.003 Figure S1. Construction and interpretation of the directional shifts of vectors describing changes in foliar phytochemical concentrations, foliar phytochemical content, and individual foliar biomass relative to control references. Dotted lines represent no change in phytochemical content (X-axis) or concentration (Y-axis) relative to control. Dashed line represents relative foliar biomass for the control, vector endpoints falling to the left or right of the dashed line represent decreased or increased foliar biomass, respectively. Interpretations of the six more common vector endpoints are shown. Figure is replicated from Couture 2011. 6 Parent: Control Damaged 25 A 20 (% dw) Total phenolic glycosides Figure S2. Total phenolic glycoside concentrations (% dry weight (%dw)) in foliage of seedlings from maternal herbivory or maternal control trees, averaged over genotypes within each taxon. NL is narrowleaf. Error bars represent +/- 1 standard error (SE) from the mean. B 15 C 10 5 0 NL F1 Fremont 7 Figure S3. Condensed tannin concentrations (% dw) in seedlings of maternal herbivory versus control trees within each taxon. Error bars represent 1 SE from the mean. Note: Fremont is shown for visual comparison only, and is not included in the statistical analysis (see details in Materials and Methods section). 1.00 Control Herbivory A A 0.75 (% dw) Condensed tannins Maternal: 0.50 B 0.25 0.00 NL F1 Fremont 8