chapter 18 how chemicals mix
advertisement

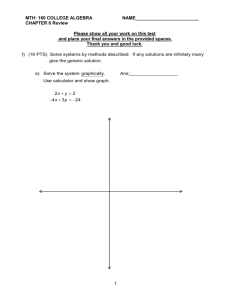
CHAPTER 18 HOW CHEMICALS MIX Multiple Choice Identify the choice that best completes the statement or answers the question. ____ 1. A combination of two or more substances in which they no longer retain their chemical properties is called a(n) _____. a. heterogeneous mixture d. mixture b. compound e. periodic trend c. suspension ____ 2. The following image represents which kind of matter? a. a mixture b. an element c. a compound ____ 3. The following image represents which kind of matter? a. an element b. a compound c. a mixture ____ ____ d. none of the above e. all of the above 4. Which of the following is a mixture? a. salt b. iron c. gold d. none of the above e. all of the above d. helium e. air 5. How does a suspension differ from a solution? a. A suspension is a heterogeneous mixture consisting of different phases whereas a solution is a homogeneous mixture consisting of a single phase. b. The difference between a suspension and a solution can only be determined by chemical means. c. Although a solution and suspension are both homogeneous mixtures, only the components of a suspension will separate by spinning the mixture in a centrifuge. d. A suspension is a heterogeneous mixture whose components can be separated by simple filtration. A solution is a homogeneous mixture which cannot be separated by simple filtration. ____ 6. Swimming pool water is best classified as a a. heterogeneous mixture b. homogenous mixture c. pure element d. pure compound ____ 7. Which of the following is a pure substance? a. orange juice b. cooking oil c. duct tape d. salt water e. baking soda ____ 8. In a solution made from one teaspoon of sugar and one liter of water, which is the solute? a. sugar d. both sugar and water b. the teaspoon e. none of the above c. water ____ 9. In a solution of 77 percent nitrogen and 23 percent oxygen, which is the solvent? a. nitrogen d. neither b. oxygen e. Gases cannot form solutions. c. both ____ 10. A sample of steel is composed of 5 percent carbon and 95 percent iron. Which is the solvent? a. carbon d. Steel is not a solution, it is a mixture. b. steel e. A solid cannot be a solvent. c. iron ____ 11. Which of the following solutions is the most concentrated? a. one liter of water with 1 gram of sugar b. one liter of water with 2 grams of sugar c. one liter of water with 10 grams of sugar d. one liter of water with 5 grams of sugar e. They all have the same volume. ____ 12. How is the solubility of a gas affected by temperature? a. As temperature goes down, the solubility goes down. b. As temperature goes up, the solubility stays the same. c. As temperature goes down, the solubility goes up. d. As temperature goes up, the solubility goes up. True/False Indicate whether the statement is true or false. ____ 13. The elements of a compound can be separated from each other by physical means. ____ 14. The solubility of a gas in water decreases with increasing temperature. ____ 15. A solution is saturated when no more solute can dissolve in that solution. Matching a. ELEMENT b. HOMOGENEOUS MIXTURE c. HETEROGENEOUS MIXTURE d. COMPOUND ____ 16. Aluminum ____ 17. Air (in a small space) ____ 18. Distilled water ____ 19. Tap water ____ 20. Table sugar ____ 21. Tea with sugar ____ 22. Italian salad dressing ____ 23. cabon dioxide ____ 24. pizza ____ 25. soft drink ____ 26. 14 kt gold ____ 27. stainless steel SELECT THE BEST ANSWER- NOT ALL CHOICES WILL BE USED a. dissolving f. mixture b. distillation g. saturated solution c. heterogeneous mixture h. solute d. homogeneous mixture i. solvent e. insoluble j. unsaturated solution ____ 28. a solution that is capable of dissolving additional solute ____ 29. a mixture in which the various components can be seen as individual substances ____ 30. a solution containing the maximum amount of solute that will dissolve in its solvent ____ 31. the component in a solution that is present in the largest amount ____ 32. a purifyling process in which a vaporized substance is collected by exposing it to cooler temperatures over a recieving flask, which collects the condensed purified liquid ____ 33. the process of mixing a solute in a solvent to produce a homogeneous mixture ____ 34. a mixture in which the various compounents are so finely mixed that the composition is the same throughout. ____ 35. not capable of dissolveing to any appreciale extent in a given solution. Essay 36. How might you separate a mixture of sand and salt? How about a mixture of iron and sand? CHAPTER 18 HOW CHEMICALS MIX Answer Section MULTIPLE CHOICE 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. ANS: ANS: ANS: ANS: ANS: TOP: ANS: TOP: ANS: TOP: ANS: ANS: ANS: ANS: TOP: ANS: TOP: B PTS: 1 DIF: 1 A PTS: 1 DIF: 1 B PTS: 1 DIF: 1 E PTS: 1 DIF: 2 C PTS: 1 DIF: 1 The chemist's classification of matter B PTS: 1 DIF: 1 The chemist's classification of matter E PTS: 1 DIF: 2 The chemist's classification of matter A PTS: 1 DIF: 1 A PTS: 1 DIF: 1 C PTS: 1 DIF: 1 C PTS: 1 DIF: 1 Concentration is given as moles per liter C PTS: 1 DIF: 1 Solubility measures how well a solute dissolves TOP: TOP: TOP: TOP: Most materials are mixtures Most materials are mixtures Most materials are mixtures Most materials are mixtures TOP: Making solutions TOP: Making solutions TOP: Making solutions TRUE/FALSE 13. ANS: F PTS: 1 DIF: 1 14. ANS: T PTS: 1 DIF: 1 TOP: Solubility measures how well a solute dissolves 15. ANS: T PTS: 1 DIF: 1 TOP: Solubility measures how well a solute dissolves MATCHING 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: A B D B D B C D C B B B PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: 1 1 1 1 1 1 1 1 1 1 1 1 TOP: Most materials are mixtures 28. 29. 30. 31. 32. 33. 34. 35. ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: J C G I B A D E PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: 1 1 1 1 1 1 1 1 ESSAY 36. ANS: Add the mixture of sand and salt to some water. Stir, and then filter the sand. Rinse the sand several times with fresh water to make sure that all of the salt has been removed. Collect all the salty water and evaporate away the water. The residue that remains will be the salt. After the sand dries, you’ve got just the sand. For a mixture of iron and sand, take advantage of the fact that only iron is attracted to a magnet. PTS: 1 DIF: 2 TOP: Most materials are mixtures