SPS4 Periodic Table (SPS4periodictable)
advertisement

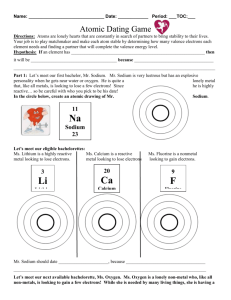
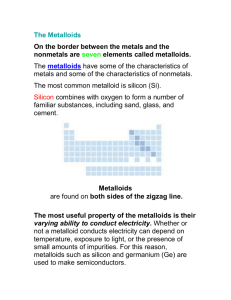
SPS4 Periodic Table (SPS4periodictable) Name:_____________________________________________ Date:________________________ 1. As you move from left to right across a row of elements in the periodic table, what happens to the number of neutrons in a typical atom? A. It stays the same. B. It increases. C. It decreases. D. It decreases until you reach the middle and then it increases. 2. Which of the following describes a particle that contains 36 electrons, 49 neutrons, and 38 protons? A. an ion with a charge of 2B. an ion with a charge of 2+ C. an atom with a mass of 38 amu D. an atom with a mass of 49 amu 3. Which of the following is the same for both hydrogen and potassium? A. atomic mass B. total mass of neutrons C. number of valence electrons D. number of filled energy levels . 4. The bar graph below represents four elements and their relative atomic numbers. What would be the most likely positioning of these unknown elements in the periodic table? A. B. C. D. . 5. Compare the elements in the halogen family. Which statement is true regarding a progression from fluorine to iodine? A. The elements progressively change phase from gas to liquid to solid. B. The elements form progressively higher charged ions. C. The elements become progressively less reactive. D. The elements decrease in density. 6. Which of these elements would you expect to be solid at room temperature? A. N B. O C. Ca D. Rn 7. As you move from left to right across the periodic table, characteristics of the elements change as well. Which is the correct sequence, moving from left to right? A. metal - metalloid - nonmetal C. metalloid - metal - nonmetal B. metal - nonmetal - metalloid D. nonmetal - metalloid - metal 8. Locate the following four elements in the periodic table. Which element has the most valence electrons? Fe, Hg, Sn, Xe A. Fe B. Hg C. Sn D. Xe 9. Which statement best describes the elements in the Noble gas family? A. Noble gases are highly flammable. B. Noble gases combine readily with reactive metals. C. Noble gases react with oxygen to form gaseous molecules in the ozone. D. Noble gases are nonreactive and have a valence number of "0". 10. Which of these statements is true about sodium (Na)? A. Sodium is an inert gas and does not combine readily with other elements. B. Sodium is a reactive nonmetal and combines readily with other nonmetals. C. Sodium is less reactive than magnesium (mG0 when combining with nonmetals like oxygen (O). D. Sodium is less reactive than potassium (K) when combining with nonmetals like oxygen (O). 11. Which of these statements is true? A. Metals tend to lose electrons and form positive ions. B. Nonmetals tend to lose electrons and form positive ions. C. Metalloids do not form compounds readily. D. Gases share electrons and form positive ions. 12. Elements from which two groups in the periodic table would most likely combine with each other to form an ionic compound? A. 1 and 2 B. 16 and 17 C. 1 and 17 D. 17 and 18 13. Three elements, X, Y, and Z, have consecutive increasing atomic numbers. If element X is a noble gas, what will be the symbol for the ion of element Z in its compounds? A. B. C. 14. The alkali metals are located in which group of the periodic table? A. 1 B. 2 C. 3 D. 4 15. Which of the following properties decreases from left to right across a period? A. Atomic number B. Electronegativity C. Atomic radius D. Ionization energy Answer Key 1. B) It increases. 2. B) an ion with a charge of 2+ 3. C) number of valence electrons 4. D) 5. A) The elements progressively change phase from gas to liquid to solid. 6. C) Ca 7. A) metal - metalloid - nonmetal 8. D) Xe 9. D) Noble gases are nonreactive and have a valence number of "0". 10. D) Sodium is less reactive than potassium (K) when combining with nonmetals like oxygen (O). 11. A) Metals tend to lose electrons and form positive ions. 12. C) 1 and 17 13. 14. A) 1 15. C) Atomic radius