csi table
advertisement

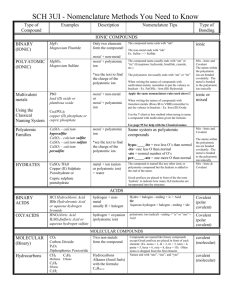
Chemical Nomenclature: Who Are You? Ionic Compounds In order to name a chemical compound we must have some knowledge of the type of compound we are naming. We will begin with Ionic Compounds as they have the simplest rules and once we master the techniques of naming ionics we can move on to more complicated Covalent Molecules. *Generally an ionic compound is made of a metal and a non-metal. *If there is any doubt use the electronegativity difference to be sure (EN) (we will revisit this later) *If a polyatomic ion is present the compound is ionic Stepwise Method for Naming Ionic Compounds 1. Name the metal first (ie. NaCl, sodium chloride) 2. The name of the nonmetal has -ide added (ie: NaCl sodium chloride) 3. If the metal has more than one possible charge, we must indicate which oxidation state (charge) is present using the Stock Method; it indicates the ion by writing the charge in roman numerals (ie: FeCl2 Iron (II) chloride). Polyatomic Ions A polyatomic ion is a molecule that, rather than being neutral carries a charge (either positive or negative). There are many polyatomic ions, all that we will examine can be found on your Magic Sheet. A sample of some polyatomics can be found in the table to the right. Naming a Compound with a Polyatomic Ion The process is nearly identical to a binary ionic. Consider NaNO3: 1. Name the metal, sodium (Na) 2. Name the polyatomic ion, nitrate (NO3-) 3. Name the compound, sodium nitrate Compound FeF2 FeF3 Hg2Br2 HgBr2 Name of Ion cyanide sulfate phosphate carbonate chromate silicate nitrite nitrate hydroxide ammonium Stock Method iron (II) fluoride iron (III) fluoride mercury (I) bromide mercury (II) bromide Formula CNSO4-2 PO4-3 CO3-2 CrO4SiO3NO2NO3OHNH4+ Charge -1 -2 -3 -3 -1 -1 -1 -1 -1 +1 Using a Polyatomic Ion to Determine the Charge on a Metal Consider Fe(OH)2 1. Use the polyatomic ion to determine the charge of the metal, OH- is -1, 2 OH-‘s in the formula therefore Fe must be +2 2. Name the metal using Stock Method, iron (II) (Fe) 3. Name the anion, hydroxide (OH-) 4. Name the compound, iron (II) hydroxide Number Prefix 1 monoHydrates 2 diMany ionic compounds also have water molecules attached to the formula; these do not 3 triaffect the name of the ionic compound; however we must have a way to account for 4 tetrathem within the name. We refer to them as hydrates. We use the covalent prefixes to 5 pentaindicate the number of H2O molecules present in a hydrate. Covalent prefixes 6 hexa. 7 heptaConsider CuSO4 5H2O 8 octacopper (II) sulfate pentahydrate 9 nona10 decaCovalent Molecules When we name a covalent molecule the process is very similar to that used for an ionic compound. The difference lies in the use of prefixes. ***Covalent molecules are typically made up of two or more nonmetals and we cannot determine the number of atoms in a covalent compound simply by naming the first and second element.*** For example: carbon (C) and oxygen (O) can combine to form CO and CO2 (also CO32- but we will neglect this for the time being). If we were to use the ionic naming system we would get for each case carbon oxide, which does not tell the two molecules apart. To this end we need to use the prefixes: mono, di, tri, tetra etc. To name covalent compounds use the following method prefix + first element + prefix + root of second element + ide (the prefix "mono" is assumed, and therefore dropped) Exceptions: 1. H2O is water, not dihydrogen oxide. 2. NH3 is ammonia, not nitrogen trihydride. 3. CO is carbon monoxide, but in all other cases, the mono is dropped. Examples 1. SO2 (sulfur dioxide) 2. Si3P6 (trisilicon hexaphosphide) 3. BF3 (boron trifluoride) 4. CO2 (carbon dioxide) 5. S5F (pentasulfur fluoride) Naming Acids, that pesky H atom! An acid is a special kind of compound. When it is dissolved in water, an acid releases a hydrogen ion (H+) into solution. Even though many acids are made from two non-metals (H and F, Cl, Br, I) we treat them as ionic compounds. We can tell a compound is an acid by indicating its state: o HCl(g) is hydrogen chloride gas o HCl(aq) is hydrochloric acid because (aq) means aqueous or dissolved in water To name an acid if it is binary (having hydrogen and either F-, Cl-, Br- or I-) 1. hydro ________ic acid (fluor, chlor, brom, iod) 2. HBr(aq) is hydrobromic acid To name an acid with a polyatomic ion 1. Replace the suffix “ate” with “ic” and add “acid” to complete the name 2. Replace the suffix “ite” with “ous” and add “acid” to complete the name 3. HNO3 (aq) (hydrogen nitrate, becomes “nitric acid”) 4. HNO2 (aq) (hydrogen nitrite becomes “nitrous acid”) Complete each of the following exercises for practice Name Formula CuSO4 . 5H2O Sodium hydroxide K3As Cupric chloride FeCO3 Sodium phosphate tetrahydrate Sb2(Cr2O7)3 Rubidium selenide Ca3P2 Cobalt (III) nitrate Na3PO3 Manganese (II) arsenide Cs2C2O4 Bismuth (V) phosphate Hg(NO3)2 Barium bromide Li2SiO3 Palladium (IV) sulfite Na2S2O3 Strontium telluride KMnO4 Mercurous cyanide MgSO4 . 6H2O Sodium bicarbonate NH4ClO4 CS2 N2H4 SiF6 dinitrogen monoxide P2O5 oxygen difluoride PCl3 SiO2 sulfur trioxide hydrochloric acid HBr (aq) HI (aq) HNO2 (aq) HNO3 (aq) perchloric acid H2SO4 (aq)