2012
advertisement

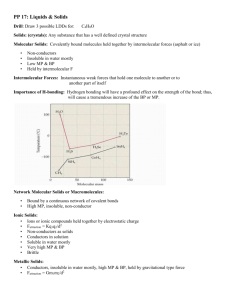
1 a) Define the following terms that can commonly be found in a binary phase diagram; the liquidus,
solidus, solvus lines and the Eutectic point.
{4}
b) Two phase diagrams are shown below:
(1)
(2)
A binary system with two solid phases, and is shown in Phase diagram 1. is the solid which is
rich in material A and is similarly the solid which is B rich. Phase diagram 2 shows a simplified
version of the Forsterite (Mg2SiO4) – Quartz (SiO2) system with its intermetallic compound Enstatite
(MgSiO3). Forsterite and Enstatite have orthorhombic crystal structures whilst quartz is trigonal.
(i) Identify the phases present in regions i, ii, iii and iv in each of the phase diagrams.
{4}
(ii) Using phase diagram (1) what is the composition of any phases and their relative weight
fractions at 300°C after cooling a 40 wt. % mixture from the liquid state?
{2}
(iii) Using suitable sketches and phase diagram (2) describe the compositions and structures
that form when cooling a 32wt. % SiO2 material from the melt.
{7}
c)
(i) A neutron powder diffraction experiment is performed on a solid solution with 10 wt. %
SiO2 as a function of increasing temperature. Describe how the diffraction profile would
change over the temperature range 1500°C to 1900°C.
{6}
(ii) How would the data be different if they were measured using x-rays?
{2}
2 a)
i) Using suitable sketches give examples of 4 types of point defect that can be found in a
single crystal. Briefly describe how they are formed.
{6}
b) A crystal of BiBaO3 is prepared by the float-zone method. This material has the perovskite
structure which is cubic at high temperatures.
i) Describe, with the aid of a simple sketch, how a single crystal is formed using this growth
method. Discuss how the impurity profile can be modified by repeated thermal cycling of
the crystal.
{6}
ii) Describe the mechanism by which the cubic phase would distort if subjected to a shear
force and the effect of any point defects.
{4}
c) Recently reported resistivity measurements on this material show an abrupt increase in the
resistance of the material at 160°C. This is attributed to a shift from a low temperature tetragonal
phase which is ferroelectric to the high temperature paraelectric cubic phase.
i) Sketch the structure of this material below and above the transition temperature. Clearly
show how an electric dipole could be generated in this material and describe why the high
temperature phase is paraelectric.
{5}
d)
i) As a material crosses a phase boundary it undergoes a phase change. These transitions
are classified as first or second order. Describe clearly what these terms mean and the
differences between them. Give an example of each clearly stating what you are using as the
ordering parameter, .
{4}
ANSWERS:
a) [Bookwork]
Liquidus line
Solidus line
Solvus line
Eutectic Point
The line separating the field of all liquid from that of liquid plus crystals
The line separating the field of all solid from that of liquid plus crystals
The solubility limit separating a solid from a mixed solid phase
Liquid to 2 solid phases
{1 mark each – total 4}
b) [Unseen]
Phase Diagram 1
i
Liquid + alpha
ii
Liquid + beta
iii
Solid solution of alpha and beta
iv
Alpha & beta
Phase Diagram 1
i
Liquid + Mg2SiO4
ii
Mg2SiO4 + MgSiO3
iii
MgSiO3 + liquid
iv
MgSiO3 + SiO2
{½ mark each total of 4}
Composition:
Alpha Phase ≈10wt. %
Beta Phase ≈90wt. %
Weight Fraction:
W
W
C C0
C C
50
62.5%
80
C0 C 30
37.5%
C C 80
{2}
(iii) Remain all liquid until it reached the liquidus temperature at about 1600o. Crystals of fosterite
would begin to precipitate and the composition of the liquid would begin to change along the
liquidus toward the peritectic, P. At P (1580°C), all of the Fosterite previously precipitated would
react with the liquid to produce crystals of Enstatite. After this reaction has run to completion there
would still be some liquid. With decreasing temperature, more crystals of En would form, and the
liquid composition would change along the liquidus toward the eutectic. At E a lamellar structure of
quartz and enstatite would form from the remaining liquid.
{5}
2 marks for sketches:
c) [Unseen/interpretation]
Stage 1: Solid solution of Fo and En. Expect to see peaks from both phases – orthorhombic so no
systematic absences. As neutrons expect broad peaks to high 2.
{1}
Stage 2: The mixture will begin to melt at the peritectic temperature. At this point En will melt to
crystals of Fo plus liquid. Neutron peaks of the En phase will disappear. Also expect to see an
amorphous liquid scattering start to appear.
{3}
Stage 3: Steady reduction in Fo peaks as the temperature rises with a concomitant increase in the
liquid scattering.
{1}
Stage 4: Only liquid. No long-range order. All diffraction peaks gone, only remain a liquid scattering
profile.
{1}
(ii) With x-rays expect to see:
Sharper peaks, sin/, enhanced fall off-at higher temperature, greater attenuation by the liquid.
{ 1 mark each, maximum 2}
Question 2
a) [Bookwork] 4 from:
Vacancy
Missing atom in lattice
Interstitial
Additional atom position not on a lattice point
squeezed between the normal atom positions
Substiutional Small
Replacement atom that is smaller than the host
Substiutional Large
Replacement atom that is larger than the host
Frenkel
Ionic defect. Cation displaced from lattice position
to an interstitial site – charge neutral.
Schottky
Ionic defect. Vacancies created by removal of
charged ion – leaves a net charge.
{4 Marks for descriptions and 2 for sketches}
b i) [Bookwork/interpretation]
Halogen bulbs provide a focused heat source to
melt a pre-loaded powder sample.
Sample rotated and moved slowly through hot
zone. A liquid zone is passed through a free
standing polycrystalline material.
Sample size limited by surface tension of liquid.
{3 Marks for descriptions and 1 for sketch}
[unseen]
IMPURITIES: For materials with k<1 impurities stay in the liquid and so move through the crystal to
the bottom. Repeated cycling drives the impurities to one end.
{2}
ii) [Unseen]
Deformation is induced by the movement of dislocations – an edge defect.
{2}
Shear force moves the edge dislocation through the crystal.
{2}
c [bookwork/interpretation – unseen BaBiO3]
Ba – corner atoms
Oxygen octahedral
Centre atom Bi
Cubic
Tetragonal
{3}
Electric dipole induced by breaking of the centrosymetry of the cubic phase. Induced moment
points along the vertical axis.
{1}
At high temperatures, the centre Bi atom oscillates in the oxygen cage giving a time averaged
moment of zero.
{1}
d) [bookwork/interpretation]
1st Order – discontinuity in the order parameter. The order parameter is the 1st order derivative of
the Gibbs free energy with respect to a thermodynamic variable. Latent heat.
{1}
2nd Order – discontinuity in the first derivative of the order parameter (or the 2nd derivative of the
Gibbs free energy). No latent heat.
{1}
1st order example: Boiling water – density, volume
2nd order example: Magnetic ordering – magnetisation, magnetic neutron diffraction peak
intensity etc.
2 marks available for suitable examples