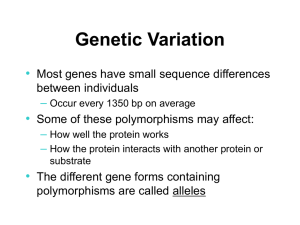
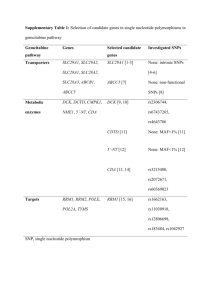
Supplementary Tables Impact of Genetic Polymorphisms of ABCB1
advertisement

Supplementary Tables Impact of Genetic Polymorphisms of ABCB1 (MDR1, P-glycoprotein) on Drug Disposition and Potential Clinical Implications: Update of the Literature Clinical Pharmacokinetics Stefan Wolking,1 Elke Schaeffeler,2,3 Holger Lerche,1 Matthias Schwab,2,4# Anne T. Nies2,3 1 Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research, University of Tübingen, Hoppe-Seyler Strasse 3, 72076 Tübingen, Germany 2 Dr. Margarete Fischer-Bosch Institute of Clinical Pharmacology, Auerbachstr. 112, 70376 Stuttgart, Germany 3 University of Tübingen, Germany 4 Department of Clinical Pharmacology, University Hospital Tübingen, Auf der Morgenstelle 8, 72076 Tübingen, Germany # Corresponding author Email: Matthias.Schwab@ikp-stuttgart.de Tel: +49 711 8101 3700 Fax: +49 711 85 92 95 1 Supplementary Table 1 Extended list of effects of ABCB1 polymorphisms on pharmacokinetics Haplotypes Other SNPs C3435T Doxorubicin G2677T/A Anticancer Docetaxel C1236T Drug Analgetic Fentanyl Methadone Kim et al. [1] Levran et al. [2] X X X X X X 196 patients, post-op 11,15,33 109 former heroin addicts 44,47,48 Korean Jewish ancestry D D Fajac et al. [3] X X X X 8,10 86 patients, breast cancer Mixed AUC Kim et al. [4] X X X 216 patients, breast cancer Korean Lal et al. [5] X X X 62 patients, advanced breast cancer 151 patients, breast cancer Asian Article Voon et al. [6] X X 51 No. of patients Ethnicity Parameters Effect NS 1236TT/2677TT/3435TT: D Asian Erlotinib Fukudo et al. [7] X X X 88 patients, NSCLC Japanese Imatinib Gurney et al. [8] X X X UK Irinotecan Han et al. [9] Zhou et al. [10] X X X X X X Paclitaxel de Graan et al. [11] 22 patients, gastrointestinal tumor 107 patients, NSCLC 29 patients, nasopharyngeal carcinoma 261 cancer patients Caucasian Japanese X Fransson et al. [12] Green et al. [13] Jiko et al. [14] X X X X X X 33 patients, gynecological cancer 38 patients, ovarian cancer X X 16 patients, urogenital Korean Asian mainly Caucasian Caucasian 3435TT: AUC in premenopausal women AUC, AE, OS 3435TT: OS, AUC for docetaxel, neutropenia for docetaxel CL, Cmax, 1236CC/2677GG/3435CC: AUC, AUC Cmax, CL AUC, CL, 2677T: CL; rs2235047G: Cmax, t1/2, OS, neutrophils, OS PFS, neutrophil count C0, CL, NS response, AE CL/F 1236TT, 2677TT, 3435TT: CL/F, toxicity-related dose reduction AUC, CL 2677TT/3435TT: AUC Cmax, AUC, 3435CC: Cmax CL, t1/2 CL, AUC, NS Cmax, C0, response, AE CL 2677TT: CL AUC, Cmax, CL, AE CL, C0/D 2677GA: CL NS 2 cancer Nakajima et al. [15] Sissung et al. [16] X X X X X X Sunitinib Vincristine Mizuno et al. [17] Guilhaumou et al. [18] X X X X X X Bicalutamide Antiepileptic Carbamazepine Kim et al. [19] X X X Meng et al. [20] Ozgon et al. [21] Puranik et al. [22] X X X X X X X X Seo et al. [23] X X X X Zhu et al. [24] Kang et al. [25] Lovric et al. [26] X X X X X Gabapentin Lamotrigin Phenobarbital Basic et al. [27] X X Phenytoin Valproate Ponnala et al. [28] Turgut et al. [29] X Putnam et al. [36] X X X X X 26 patients, advanced solid tumors 19 patients, renal carcinoma 20 children, solid tumor X Japanese Caucasian AUC AUC, t1/2, 3435T: AUC 2677T, 3435T: neutrophils Japanese Caucasian AUC24, AUC4 C, C intracellular, AE C NS NS 27 healthy subjects Korean 41,45 84 patients, epilepsy 97 patients 90 patients, epilepsy Chinese Turkish Mixed 8 210 patients, epilepsy Japanese 210 patients, epilepsy 30 healthy subjects 222 patients, epilepsy Chinese Korean Caucasian 60 patients, generalized epilepsy 127 patients, epilepsy 104 patients, bipolar disorder Caucasian Mixed Turkish C0 3435TT: C0 C0/D, response NS CL, t1/2 1236T: CL (in African Americans) CL/F, 2677TT, 3435TT, responder 1236T/2677T/3435T: resistance status C0/D 3435CT: C0/D Cmax, AUC, t1/2 NS C0, C0/D 1236CC, 1236C/2677G/3435C: C0/D CSF 3435CT, 3435TT: CSF C concentration C0 3435CC: lower C0 C0 NS X 74 HIV patients Caucasian C0 3435CC: C0 X 118 HIV patients Caucasian C0 3435T: C0 35 HIV patients 20 Healthy subjects 18 healthy subjects 11 patients, cystic fibrosis/ 11 controls 18 healthy subjects Caucasian Chinese Chinese Caucasian C0 Cmax CL, C0, Cmax CL 2677GG: C0 2677TT/3435TT: Cmax 1236CC: C0 ,Cmax 3435TT: CL Caucasian Cmax, AUC, CL NS X X X Antiinfectious Atazanavir Rodriguez-Novoa et al. [30] Rodriguez-Novoa et al. [31] Atazanavir/Ritonavir D’Avolio et al. [32] Azithromycin He et al. [33] Cloxacilline Yin et al. [34] Dicloxacilline Beringer et al. [35] 8 X X X X X X NS 3 Efavirenz Elens et al. [37] Mukonzo et al. [38] X X Mukonzo et al. [39] Ngaimisi et al. [40] Efavirenz/ Lopinavir Winzer et al. [41] Efavirenz/ Fellay et al. [42] Nelfinavir Indinavir Curras et al. [43] Saquinavir Valacyclovir Antipsychotic Clozapine Olanzapine Levisulpiride Risperidone African African Caucasian Caucasian CL/F, F, AUC C0 C12, C0 C0 c.*193G: F c.*193G: C0 NS 3435TT: C0 C0, CL, C0/D 3435CC: C0/D 28 HIV patients South American Caucasian 103 HIV patients Mixed 113 HIV patients 28 HIV patients Brazilian Caucasian 152 HIV-infected children 8 healthy subjects Mixed Caucasian X 200 healthy subjects X 12,20,21, 121 healthy subjects 23,26,28, 30,36,38, 53 53 99 HIV patients 53 494 HIV patients 43/ 32 HIV patients 69/ 54 HIV patients X X X X X X X X X X X Saitoh et al. [48] Frohlich et al. [49] X X X X X Zhang et al. [50] X X X Consoli et al. [51] Ghotbi et al. [52] Cho et al. [53] Gunes et al. [54] X X X X X X X X X X X X X X X X X Jovanovic et al. [55] Kastelic et al. [56] Xiang et al. [57] F 1 X X X X Solas et al. [44] Lopinavir/ Ma et al. [45] Atazanavir Lopinavir/ Ritonavir Estrela et al. [46] Nelfinavir Colombo et al. [47] African c.*193G: accumulation in peripheral mononuclear cells c.*193G: F X X 50 HIV patients 21 HIV-infected children X X X Caucasian C0, CL, 3435CT vs CC: absorption rate absorption rate constant constant C0 NS NS 3435TT: cellular AUC Chinese C0 cellular C, AUC CL/F AUC, Cmax, tmax, t1/2, CL AUC, Cmax X 60 patients, psychosis 121 schizophrenic patients 58 healthy subjects 46 patients, schizophrenia Caucasian Caucasian Korean Caucasian D, C0 AUC AUC, Cmax C0/D X 83 patients, psychosis Caucasian C0/D, side effects 59 patients, psychosis 23 healthy subjects Caucasian Chinese C0, response Cmax, tmax, AUC, CL/F, t1/2 2677GG, 3545CC: C0, D NS 2677TT, 3435TT: AUC, Cmax 1236T/2677T/3435T: C0/D of 9'hydroxyrisperidone 2677TT, 3435TT: C0/D; 2677TT/3435TT: extrapyramidal side effects NS 2677GA: tmax 23 NS NS NS 4 Quetiapine Cardiac Amlodipine Yasui-Furukori et al. [58] Bakken et al. [59] X Kim et al. [60] X Aarnoudse et al. [62] X 85 schizophrenic patients Japanese C0 NS 289 schizophrenic patients Caucasian C0/D NS X 26 healthy subjects Korean Chinese 2677TT/3435TT: AUC, Cmax, CL/F 3435TT: CL/F X 60 patients; essential hypertension 195 patients, digoxin user CL/F, AUC, Cmax CL/F, AUC Caucasian C0 50 healthy subjects Caucasian AUC4, Cmax, tmax AUC4, Cmax 1236T, 2677T, 3435T, 1236T/2677T/3435T: C0 NS X Zuo et al. [61] Digoxin X X X X X Gerloff et al. [63] X X Johne et al. [64] X X X 24 healthy subjects Caucasian Kurata et al. [65] Verstuyft et al. [66] Verstuyft et al. [67] X X X X X X 15 healthy subjects 32 healthy subjects 12 healthy subjects Japanese Caucasian Caucasian X X X 20 healthy subjects Chinese 58 healthy subjects 19 healthy subjects Turkish Chinese Xu et al. [68] X 23 Losartan Telmisartan Yasar et al. [69] Guo et al. [70] Verapamil Pan et al. [71] X X X 10 healthy subjects Korean Zhao et al. [72] X X X 24 healthy subjects Chinese X X X 81 renal recipients D, C0/D 2677GG, 2677G/3435C: C0/D 42 liver recipients mainly Caucasian Caucasian C0/D 103 renal recipients 70 renal recipients 29 healthy subjects Chinese Korean Korean 65 cardiac recipients Caucasian D, AUC12/D C0 Cmax, AUC12, tmax, t1/2 D 3545TT: C0/D 1-3 days post transplantation, not beyond NS NS NS 150 liver recipients Caucasian C0/D, C0, C Immunosuppressants Tacrolimus Anglicheau et al. [73] Bonhomme-Faivre et al. [74] Cheung et al. [75] Cho et al. [76] Choi et al. [77] Diaz-Molina et al. [78] Elens et al. [79] X X 3435TT: Cmax, AUC4; 2677G/3435T: AUC CL, F 2677TT/3435TT: CL, F AUC4, AUC24 3435TT: AUC4, AUC24 AUC4, AUC24, 3435TT: AUC4, AUC24 CL, Cmax AUC4, Cmax, 1236TT/2677TT/3435TT: AUC4, tmax Cmax, tmax urine C NS Cmax, tmax, t1/2, NS AUC, CL AUC, Cmax, NS tmax Cmax, CL, 2677TT/3435TT: AUC AUC X 8 X X X X X X X X X X X X X X X 10,23 NS 1199A, 1236CC, 2677GG: C 5 206 renal recipients 103 renal recipients 98 liver recipients Mixed Caucasian Caucasian liver C0/D D, CL/F, C0 C0, C0/D, D liver 2677GG, 3435CC: C0/D NS NS Japanese Japanese C0/D C0/D NS NS Mixed C0, AUC NS X 69 liver recipients 181 liver recipients, 114 donors 19 renal transplant candidates 51 paediatric liver recipients Caucasian D X 136 renal recipients mainly Caucasian Japanese G2677TT, 3435TT, 1236T/2677T/3435T: D NS D, C0, C0/D, acute rejection C0/D NS Korean C0 Caucasian Fredericks et al. [80] Gervasini et al. [81] Gomez-Bravo et al. [82] Goto et al. [83] Goto et al. [84] X X X X X X X X X X X X X X Haufroid et al. [85] X X X Hawwa et al. [86] X X Hesselink et al. [87] Hosohata et al. [88] X X Jun et al. [89] X X Kuypers et al. [90] X X Kuypers et al. [91] Li et al. [92] X X X X Li et al. [93] X X López-Montenegro et al. [94] MacPhee et al. [95] MacPhee et al. [96] Mai et al. [97] Min et al. [98] Mourad et al. [99] X X Op den Buijsch et al. [100] X X X 63 living-donor liver recipients 506 liver/ renal recipients, 62 donors 95 renal recipients NS Caucasian Chinese Chinese D, C0/D NS X 304 renal recipients 73 liver recipients, 73 donors 70 liver recipients, 70 donors 35 renal recipients C0/D, NS AUC12/D, nephrotoxicity, acute rejection, survival. D, AUC, C0 NS CL/F NS Caucasian C0/D 3435CC: C0/D X 180 renal recipients 178 renal recipients 73 renal recipients 62 renal recipients 59 renal recipients C0/D C0, C0/D C0/D C0 D, C0/D 3435CC: C0/D NS NS NS NS X 63 renal recipients Mixed Mixed Caucasian Japanese mainly Caucasian Caucasian D, C0/D, Cmax/D NS X X X X X X X X X X X 6 X X 32 liver recipients Caucasian AUC12/D D NS X X 101 liver/ renal recipients Caucasian C0, D 2677T/A: D X X 44 renal recipients D, C0, C0/D X X 216 liver recipients mainly Caucasian Chinese -129C, 2677T, 3435T: C0/D when less than 3 copies of these SNPs NS Shilbayeh et al. [105] X 38 renal recipients Tada et al. [106] X 39 renal recipients Middle Eastern Japanese X X 30 renal recipients Japanese X X X Caucasian Chinese Provenzani et al. [101] Provenzani et al. [102] Roy et al. [103] X Shi et al. [104] Tsuchiya et al. [107] Wang et al. [108] Wei-lin et al. [109] X Zhang et al. [110] Zheng et al. [111] Tacrolimus/ Sirolimus Mourad et al. [112] X Renders et al. [113] Everolimus Tacrolimus/ Cyclosporine 8,10 X X X X 91 lung recipients 50 liver recipients, 50 donors 118 renal recipients 75 lung recipients X X X X Lemaitre et al. [114] Bandur et al. [115] X X X X X Haufroid et al. [116] X X X X Hesselink et al. [117] X Loh et al. [118] X Onizuka et al. [119] X X X Singh et al. [120] X X X X D, C0/D, early renal injury t to attain target C D, AUC12/D, Cmax, tmax, t1/2, CL/F D, AUC12/D, Cmax, Cmax/D, tmax, t1/2, CL/F C0/D C0/D NS NS NS 1236TT/2677TT/3435TT: C0/D 3435CC: C0/D D, C0, C0/D D, C0/D NS NS 24 renal recipients Chinese mainly Caucasian Caucasian D, C0, C0/D NS 134 renal recipients Caucasian NS 59 cardiac recipients 407 Cy, 425 Tac renal recipients 50 Cy, 50 Tac renal recipients 110 Cy, 64 Tac renal recipients 64 Cy, 18 Tac renal recipients 21 Cy, 37 Tac patients after HCST 225 Cy, 75 Tac renal Caucasian Caucasian Caucasian D, C0, C0/D, AUC12, AUC24 CL, CL/F C0, C0/D, rejection D, C0, C0/D Mixed D, C0, C0/D NS Mixed Asian D, C0, C0/D, C2 Japanese NS South Asian 2677GG, 3435CC: C0/D C0/D NS 1236T/2677T/3435T: risk of rejection. NS NS 7 Cyclosporine Anglicheau et al. [121] X X recipients 106 renal recipients White X X 88 liver recipients 44 liver recipients Iranian Caucasian 171 renal recipients Mixed X X 8 Azarpira et al. [122] Bonhomme-Faivre et al. [123] Bouamar et al. [124] X X X Chowbay et al. [125] X X X X Crettol et al. [126] X X X X 10,23 73 renal/ lung recipients Fanta et al. [127] X X X X 23 104 renal recipients Foote et al. [128] Garcia et al. [129] Hesselink et al. [130] X X X X X X 14 cardial recipients C0, C2, rejection, renal function Mixed Asian C0, Cmax, AUC4, AUC12 Caucasian D, C0, C0/D, intracellular concentration mainly D, CL, F Finnish X X X X X X X X Press et al. X X X X Qiu et al. [135] X X X X 103 renal recipients Caucasian Caucasian Mainly White Chinese Japanese Caucasian mainly African American mainly Caucasian Chinese Taegtmeyer et al. [136] Wang et al. [137] X X X X 337 cardial recipients Caucasian C0 X X X X 112 renal recipients Chinese C0/D 18 renal recipients Mixed Cmax/D, AUC/D, tmax, CL/F Hu et al. [131] Kuzuya et al. [132] Mai et al. [133] Min et al. [134] Yates et al. [138] X X 75 renal recipients 68 renal recipients 151 cardial/ renal recipients C0/D, Cmax/D, AUC4/D, AUC12/D, t1/2 D, C0, C0/D D, C2, C2/D 8 X 8 106 renal recipients 97 renal recipients 98 renal recipients 16 healthy subjects 33 renal recipients AUC4/D, C0 C0, AE CL/F 1236C: Cmax/D, AUC4/D 3435TT: C0/D 3435TT: D, C2/D NS 1236T/2677T/3435T: AUC4, AUC12, Cmax, C0 3435T: 1.7-fold intracellular C; 1199A: 1.8-fold intracellular C 1236CC, 2677GG, 1199G/1236C/2677G/3435C: pre-hepatic ER, F 3435T: AUC4/D 3435: nephrotoxicity NS D, C0, C0/D AUC2 C0, C2, AUC12 tmax, Cmax, t1/2, AUC, CL/F 3435C: C0/D NS NS NS CL/F, AUC NS C0/D, C2/D 1236CC, 1236C/2677A/3435C: C0/D 1236CC, 12677GG, 3435CC: C0 2677TT, 1236T/2677T/3435C: C0/D 3435T: CL/F 8 Methotrexate Budesonide Kim et al. [139] Dilger et al. [140] Prednisolone Platelet Inhibition Clopidogrel Miura et al. [141] Statins Fluvastatin/ Pravastatin/ Lovastatin/ Rosuvastatin Miscellaneous Fexofenadine X X X X X X Frelinger et al. [142] X Taubert et al. [143] X Karazniewicz-Lada [144] X Keskitalo et al. [145] X X X X Drescher et al. [146] Kim 2001 [147] X X X X X 50 20 patients after HCST 21 patients, primary biliary cirrhosis 95 renal recipients Korean Caucasian CL AUC, Cmax 3435CC,CT: CL NS Japanese Cmax, AUC24 NS 160 healthy subjects, homozygous CYP2C19 60 patients, coronary artery disease 42 patients undergoing coronary angiography Mixed AUC, Cmax NS Caucasian AUC, Cmax 3435TT: Cmax, AUC Caucasian AUC, Cmax 3435TT: Cmax, AUC 20 healthy subjects Caucasian AUC NS 20 healthy subjects healthy subjects Caucasian Caucasian and AfricanAmerican Japanese Caucasian AUCinf, Cmax AUC, C0 NS 1236C/2677G/3435C: AUC Kodaira et al. [148] X 15 healthy subjects Cmax 3435TT: Cmax Pauli-Magnus et al. X 16 healthy subjects AUC, tmax NS [149] Skarke et al. [150] X X X 21 healthy subjects Caucasian AUC6 NS Rebamipide Cho et al. [151] X X 30 healthy subjects Korean AUC, Cmax NS “X” denotes that this variant was tested. “Other SNPs” are found in supplementary Table 3. AE=adverse effects, AUC=area under concentration-time curve, AUCx=AUC from time 0 to x, C=concentration, C/D=dose dependent C, C0=trough concentration, C0/D= dose dependent C0, Cmax=maximum concentration, CSF=cerebrospinal fluid, Cx=concentration at time x, CL= apparent body clearance, CL/F=oral clearance, D=required dose, F=bioavailability, NS=not significant, OS=overall survival, PFS=progression free survival, t1/2=elimination half time, tmax=time to Cmax, increase, decrease. Lansoprazole Loperamide 9 Supplementary Table 2 Extended list of effects of ABCB1 polymorphisms on pharmacodynamics G2677T/A C3435T Haplotypes Other SNPs Morphine C1236T Drug Analgetic Methadone Coller et al. [152] X X X X 10,23 Lotsch et al. [153] X X X 60 patients, opioid dependent 51 healthy subjects X 145 patients Caucasian pupil size 9h after administration pain relief 74 patients, post-op Caucasian D, AE References Campa et al. [154] Parameters Effect Australian D 1236TT/2677TT/3435TT: D Caucasian NS X X X X X X X X X X X X X Zwisler et al. [160] X X 33 healthy subjects Caucasian Zwisler et al. [161] X X 256 patients, postoperative pain Gonzales-Haba et al. X [162] X X 5-FU+ epirubicin+ cyclophosphamide Cetuximab + Irinotecan Cisplatin Vulsteke et al. [163] X X X Docetaxel Sissung et al. [166] Anticancer 5-FU/ Capecitabine Ross et al. [158] Sia et al. [159] Paule et al. [164] X Ethnicity X Oxycodon Coulbault et al. [155] Fujita et al. [156] Lotsch et al. [157] No. of patients 8,20 X Chen et al. [165] X X X X X 8 X 3435 associated with pain relief variability 2677GG/3435CC: AE 1236TT, 2677TT/3435TT: fatigue 3435TT: D Caucasian AE efficacy (24h-pain score), AE, D efficacy, AE morphine consumption, pain scores, AE efficacy (cold pressor test), AE pain relief (NRS) 141 patients, CRC Caucasian AE 991 patients, breast cancer 23 patients, refractory liver metastatic CRC 95 patients, NSCLC Caucasian survival 1236, 3435, 2677: altered risks of AE: hand-foot syndrome, leukopenia, diarrhea 2677GT: survival Caucasian outcome 3435TT: outcome Chinese response, AE 73 patients, androgen independent prostate mainly Caucasian OS 2677T: resistance; -129T/2677T/3435C: response 1236C/2677G/3435C: OS 32 cancer patients Japanese 352 patients with chronic pain 228 cancer patients Caucasian women after C-section Chinese 2677G: AE NS 3435TT: less AE; 2677TT: AE, efficacy NS 10 Tsai et al. [167] X X X Choi et al. [168] X X X 6,7 AE 92 patients, solid tumors Koreans AE, response 54 patients, SCLC Japanese response 2677GG: fever, febrile neutropenia 3435TT: neutropenia, anemia. 2677G/T: leucopenia, resistance 3435CC, 2677G/3435C: response 48 patients, pancreatic cancer 46 patients, CML Japanese outcome, AE NS Caucasian resistance Caucasian North African Chinese Japanese response, C0 response 1236TT, 1236CT, 2677TT, 3435TT: resistance 1236TT: C0, response 2677TT: response response AE 1236TT, 3435TT: resistance 3435TT: diarrhea. 250 patients, metastatic CRC White response, AUC NS 109 patients, metastatic breast cancer 43 patients, gastric cancer 111 patients, breast cancer 914 patients, ovarian cancer 26 patients, advanced solid tumor 51 patients, advanced ovarian cancer 53 patients, ovarian cancer 88 patients, renal carcinoma 96 patients, renal carcinoma Korean survival, chemoresistance PFS 3435CT: survival. 2677GG: chemoresistance 3435TT: PFS NS Caucasian response, neurotoxicity response, AE Caucasian AE NS Caucasian PFS 1199T/A: PFS Caucasian response 2677TT, 2677TA: response mainly Caucasian mainly Caucasian PFS, OS 1236TT: OS, PFS time to dose reduction 1236TT: time to dose reduction Etoposide + Cisplatin Gemcitabine Sohn et al. [169] X X Kasuya et al. [170] X X Imatinib Deenik et al. [171] X X X Dulucq et al. [172] Elghannam et al. [173] Ni et al. [174] Lara et al. [175] X X X X 90 patients, CML 96 patients, CML X X X X 52 patients, CML 651 patients, SCLC De Mattia et al. [176] X X X X Chang et al. [177] X X X Chang et al. [178] X X Irinotecan + Cisplatin Irinotecan + Leukovirin + Cisplatin Paclitaxel Paclitaxel + Carboplatin Hertz et al. [179] X X X Marsh et al. [180] X X X Sissung et al. [16] X X X Green et al. [181] X Green et al. [182] Sunitinib Beuselinck et al. [183] Beuselinck et al [184] X cancer 59 patients, breast cancer Chinese 8,16,22 23,27,29 X 23,25 X X X X X X X X Chinese Mixed NS 11 Garcia-Donas et al. [185] van Erp et al. [186] X X X X X X Temozolomide Schaich et al. [187] X X X Vinorelbin + Cisplatin Methotrexate Doxorubicin+ vinvristine+ prednsisolone Methotrexate Antidepressant Amitriptylin/ Citalopram/ Mirtazepine/ Paroxetin/ Venlafaxine Pan et al. [188] X X Citalopram Karlsson et al. [192] X X X 101 patients, renal carcinoma 219 patients, renal carcinoma 119 patients, glioblastoma 69 patients, NSCLC Caucasian AE, response NS Caucasian AE Caucasian survival Chinese response 1236T/2677T/3435T: hand foot syndrome 1236CC: survival, independent of MGMT status 3435CC, 2677G/3435C: response Zgheib et al. [189] Gregers et al. [190] X X X X X X 127 patients, ALL 522 patients, ALL Lebanese Danish AE AE 3435T and 1236T: neutropenia 3435TT: bone marrow toxicity Gregers et al. [190] X X X 522 patients, ALL Danish AE 3435CC: liver toxicity 16,35,38 39,41-47 443 patients, major depression Caucasian response (HAMD- 95 SNPs examined. Only SNPs with 17) significant response are listed. NS for mirtazapine 23 112 forensic autopsy cases positive for citalopram 54 patients, major depression 100 patients, major depression mainly Caucasian rate of lethal intoxication Turkish response (HAMD- NS 17) response (HAMD- rs1922242, rs1202184: association 17) with severity of depressive symptoms; rs1882478/rs2235048/rs2235047 /rs1045642/rs6949448 (TTTCC): remission response 1236T/2677G/3435C: response Uhr et al. [191] X Ozbey et al. [193] X X Escitalopram Lin et al. [194] X X X 3-6,11,13 14,17-19 32,37,39 40,48,5052 Paroxetin Kato et al. [195] X X X X X X Paroxetin/ Mirtazepine Mihaljevic et al. [196] Sarginson et al. [197] X X Venlafaxine Karlsson et al. [192] X X X Patients with major depression 127 patients, major depression 1,8-10,16 246 patients, major 22,23,26,27 depression 31,34,44 23 116 forensic autopsy Chinese Japanese Caucasian NS mainly Caucasian response (HAMD- NS 17) response (HAMD- Paroxetine: rs2032583C, 2235040A: 17) earlier response; mirtazapine: NS mainly rate of lethal 1236CC+CT, 3435CC+CT: 12 Fluoxetine Gassó et al. [198] Various drugs Menu et al. [199] Antiemetic Domperidone Granisetron/ Tropisetron/ Ondansetron Granisetron + Dexamethasone Ondansetron Ondansetron + Metoclopramide Ondansetron/ Granisetron Antiepileptic Carbamazepine X X X X Caucasian intoxication intoxication Caucasian response (CGII) 2677TT: response Caucasian response (HAMD- NS 17, BDI, CGII, TI) 48 patients, gastroparesis 216 cancer patients Caucasian Turkish D response (selfreport) 61G: D 3435TT: response response (control of emesis) vomiting after surgery nausea, vomiting 2677TT: response response (control of emesis) 3435TT: response Parkman et al. [200] X Babaoglu et al. [201] X X X Tsuji et al. [202] X X 64 patients, breast cancer Japanese Choi et al. [203] X X Korean X X X X X X 198 patients, postoperative 202 patients, cisplatin therapy 239 cancer patients South Asian North African South Asian Turkish responder status 2677TT: response X 314 patients, focal epilepsy 100 patients, epilepsy response 3435CC: resistance responder status NS resistant epilepsy NS Mixed responder status NS Chinese Caucasian responder status responder status NS NS Mixed responder status NS Mixed responder status NS Asian responder Status Complex haplotypic effects Perwitasari et al. [204] Zoto et al. [205] Phenytoin Subenthiran et al. [206] Ebid et al. [207] Valproic acid Haerian et al. [208] Various drugs Alpman et al. [209] Bournissen et al. [210] Dong et al. [211] Emich-Widera et al. [212] Haerian et al. [213] 10 cases positive for venlafaxine 83 children and adolescents 117 patients, major depression X X X X X X 249 patients, epilepsy X X X 39 children, epilepsy, 92 controls 3371 patients, epilepsy, (MA) 350 children, epilepsy 85 patients, epilepsy X X X X X X Haerian et al. [214] X X X X Hung et al. [215] X X X X 6755 patients, epilepsy, (MA) 7067 patients, epilepsy, (MA) 331 patients, epilepsy, 287 controls South Asian Turkish 2677TT, 3435TT: vomiting 2h post-op; NS 24h post-op 1236T/2677G/3435C: response 13 Kwan et al. [216] Leschziner et al. [217] Sanchez et al. [218] Siddiqui et al. [219] Antiinfectious Atazanavir Mefloquine Nevirapine Antipsychotic Olanzapine Risperidone Colchicine Cyclosporine X X X X X X X X X 464 patients, epilepsy 503 patients, epilepsy Chinese UK responder status responder status 2677: related to drug resistance NS responder status responder status NS 3435CC: resistance responder status NS response NS 2677TT: severe hyperbilirubinemia 1236TT, 2677TT, 3435TT, 1236TT/2677TT/3435TT: AE 3435TT: hepatotoxicity 3435T: hepatotoxicity Tan et al. [220] X 289 patients, epilepsy 315 patients, epilepsy, 200 controls 609 patients, epilepsy Manna et al. [221] X 175 patients, epilepsy Caucasian mainly Caucasian mainly Caucasian Caucasian X X 129 HIV-patients Korean AE X X 89 healthy travelers White X X 156 HIV-patients 53 HIV-patients Caucasian Mixed neuropsychiatric AE hepatotoxicity hepatotoxicity X X X X 87 schizophrenic patients Caucasian 41 schizophrenic patients Caucasian X X X X X X X X X X Park et al. [222] Aarnoudse et al. [223] Ciccacci et al. [224] Haas et al. [225] Bozina et al. [226] Lin et al. [227] X X Kuzman et al. [228] Xing et al. [229] Immunosuppressant Azathioprine X X X Mendoza et al. [230] Rustemoglu et al. [231] Saricaoglu et al. [232] Tufan et al. [233] X Grinyo et al. [234] X Hauser et al. [235] van Ahsen 2001 [236] X X 48 X X X X X X X 10,53 108 schizophrenic patients 130 schizophrenic patients Caucasian response (PANSS) 2677T, 2677TT: response response (BPRS), NS weight gain weight gain NS Chinese response (BPRS) 1236TT: response 76 patients with Crohn's disease 104 patients, Behcet's disease 97 patients, Behcet's disease 120 patients, familial mediterranean fever 237 renal recipients Brazilian resistance Turkish response 2677TT, 3435TT, 2677T/3435T: resistance 3435TT: response Turkish response NS Turkish resistance 3435C: resistance Caucasian graft rejection 97 renal recipients, 97 donors 124 renal recipients Caucasian C0/D, AE 2677T, 1236T/2677T/3435T: rejection 3435TT: nephrotoxicity Caucasian graft rejection, C0 NS 14 Cyclosporine/ Tacrolimus Methotrexate Wang et al. [237] X Woillard et al. [238] X X X X X X Hebert et al. [239] X X X X X X X X X X X Klauke et al. [240] Yamauchi et al. [241] Yanagimachi et al. [242] Zheng et al. [243] Takatori et al. [244] Samara et al. [245] 208 renal recipients 259 renal recipients, 227 donors 120 liver recipients Chinese Caucasian response, AE graft rejection NS 1236T/2677T/3435T: rejection Caucasian 106 renal recipients 17 liver recipients Caucasian Japanese chronic renal dysfunction nephrotoxicity AE 2677TT: risk; 2677G/3435C, 2677G/3435T: risk NS 2677TT: neurotoxicity Japanese neurotoxicity 1236T neurotoxicity X X X 30/ 33 patients after HSCT 117 lung recipients 124 patients, RA 159 patients, RA graft rejection response, AE response, AE 3435TT: rejection 3435TT: resistance 3435T: hepatotoxicity X 174/ 81 patients, RA Mixed Japanese Middle Eastern Caucasian remission Methotrexate: 3435T: remission X 74 children, idopathic nephrotic syndrome 31 children, ITP Chinese steroid resistance 1236T: resistance Turkish response (platelet count) NS 1034 patients, coronary DES 2208 patients, acute myocardial infarction Mixed cardiovascular event rate death of any cause, stroke or myocardial infarction cardiovascular events early/ long-term major cardiovascular events, platelet activity cardiac death, recurrent infarction platelet reactivity (VASP-PRI) ischemic events NS 7,8,53 X Methotrexate/ Sulfasalazine Steroids Drozdzik et al. [246] Methylprednisolone Akin et al. [248] X Platelet Inhibition Clopidogrel Carlquist et al. [249] X Simon et al. [250] X Singh et al. [251] X 19601 patients Mixed Su et al. [252] X MA Mixed Verschuren et al. [253] Xie et al. [254] X 1327 patients, STEMI Caucasian X 162 patients, coronary heart disease 2932 patients, acute Chinese Clopidogrel/ Chiou et al. [247] Mega et al. [255] X X X X X X mainly Caucasian mainly 3435TT cardiovascular events NS 3435T,TT: early/ long-term events NS NS Clopidogrel: 3435TT: ischemic 15 Prasugrel Clopidogrel/ Ticagrelor Statins Atorvastatin Simvastatin Wallentin et al. [256] X coronary syndrome 10285 patients, acute coronary syndrome Caucasian mainly Caucasian events ischemic/ bleeding Clopidogrel: 3435CC: ischemic events events Hoenig et al. [257] X 117 patients Caucasian Shabana et al. [258] X 50 patients, high cholesterol North African 85 patients, simvastatin user Caucasian cholesterol reduction change of HDL-, LDL-, total cholesterol cholesterol reduction Becker et al. [259] X X X X 3435CC: efficacy in LDL-C reduction 3435TT: HDL-C 1236T/2677T/3435T, 1236C/2677G/3435T: reduction of total cholesterol and LDL-C male subjects only “X” denotes that this variant was tested. “Other SNPs” are found in supplementary Table 3. AE=adverse effects, ALL, acute lymphoblastic leukemia, AUC=area under the curve, AUCx=AUC from time 0 to x, BDI=Becks Depression Inventory, BPRS=Brief psychiatric rating scale, C=concentration, C0=trough concentration, CGII=Clinician Global Impression Improvement, CML=chronic myeloid leukemia, CRC=colorectal cancer, D=required dose, DES=drug eluting stent, HAMD-17=Hamilton Depression Rating Scale, HDL-C=high density lipoprotein cholesterol, HSCT=hematopoietic stem cell transplantation, ITP=idiopathic thrombocytopenic purpura, LDL-C=low density lipoprotein cholesterol, MA=metanalysis, NSCLC=non-small cell lung cancer, NRS=numeric rating scale, NS=not significant, OS=overall survival, PANSS=Positive and negative syndrome scale, PFS=progression free survival, RA=rheumatoid arthritis, SCLC=small cell lung cancer, STEMI=ST-elevation myocardial infarction, TI=Therapeutic Index, VASP PRI=platelet reactivity index VASP, increase, decrease. 16 Supplementary Table 3 List of ABCB1 SNPs investigated in pharmacogenetic studies Position Code Amino acid or effect rs10245483 approximately 2.4 mb upstream of MDR1 2. n.a. c.-330-14566G>A rs13233308 Intron 1 3. c.-330-1517T>C rs28381796 Intron 1 4. c.-330-21247T>C rs1978095 Intron 1 5. c.-330-3655A>G rs4148732 Intron 1 6. c.-330-41A>G rs2188524 Intron 1 7. c.-145C>G rs34976462 5'UTR 8. c.-129T->C rs3213619 5'UTR 9. c.-1A->G rs2214102 5'UTR 10. c.61A->G rs9282564 Asn21Asp 11. c.117+4196T>C rs3789243 Intron 3 12. c.239C→A rs9282565 Ala80Glu 13. c.286+927G>A rs1202184 Intron 5 14. c.287-1234G>C rs10256836 Intron 5 15. c.287-1547G>A rs2520464 Intron 5 16. c.287-25G>T rs2235015 Intron 5 17. c.287-5940A>T rs1989831 Intron 5 18. c.338+123A>G rs2235018 Intron 6 19. c.338+76T>G rs2235016 Intron 6 20. c.530+139C>T rs1202168 Intron7 21. c.781A→G rs36008564 Ile261Val 22. c.1000-44G>A rs10276036 Intron 10 23. c.1199G→A rs2229109 Ser400Asn 24. c.1236C→T rs1128503 25. c.1308A>G rs35068177 Gly412= Thr436= 26. c.1350+44C>T rs2032588 Intron 13 27. c.1554+24T>C rs2235033 Intron 14 28. c.1659 G→C rs2235012 Leu554Leu 29. c.1725+38G>A rs2235013 Intron 15 30. c.1795C→T rs2235036 Ala599Thr 31. c.1887+38G>A rs28381916 Intron 16 32. c.2064+73A>G rs2235046 Intron 17 33. c.2065-76T>A rs1922242 Intron 17 34. c.2212-27A>G rs2235063 Intron 18 35. c.2320-695G>A rs12720067 Intron 19 36. c.2320-88G>A rs4728699 Intron 19 37. c.2397+557G>A rs3789246 Intron 20 38. c.2481+24G>A rs2235040 Intron 21 39. c.2481+788T>C rs10248420 Intron 21 40. c.2481+882A>T rs10234411 Intron 21 41. c.2482-236A>G rs4148739 Intron 21 42. c.2482-707A>G rs11983225 Intron 21 43. c.2677G→T/A rs2032582 44. c.2685+49T>C rs2032583 Ser893Ala/Thr Intron 22 1. 17 45. c.2686-1911T>C rs4148740 Intron 22 46. c.2686-3393T>G rs10280101 Intron 22 47. c.2786+170G>A rs2235067 Intron 23 48. c.3282+2733A>G rs6949448 Intron 26 49. c.3435C→T rs1045642 50. c.3489+1573G>A rs1882478 Ile1145= Intron 27 51. c.3489+59T>G rs2235047 Intron 27 52. c.3489+80C>T rs2235048 Intron 27 53. c.*193A>G rs3842 3′ UTR 18 Supplementary References 1. Kim K-M, Kim H-S, Lim SH, Cheong SH, Choi E-J, Kang H, et al. Effects of genetic polymorphisms of OPRM1, ABCB1, CYP3A4/5 on postoperative fentanyl consumption in Korean gynecologic patients. Int J Clin Pharmacol Ther. 2013;51:383–92. 2. Levran O, O’Hara K, Peles E, Li D, Barral S, Ray B, et al. ABCB1 (MDR1) genetic variants are associated with methadone doses required for effective treatment of heroin dependence. Hum Mol Genet. 2008;17:2219– 27. 3. Fajac A, Gligorov J, Rezai K, Lévy P, Lévy E, Selle F, et al. Effect of ABCB1 C3435T polymorphism on docetaxel pharmacokinetics according to menopausal status in breast cancer patients. Br J Cancer. 2010;103:560–6. 4. Kim H-J, Im S-A, Keam B, Ham HS, Lee KH, Kim TY, et al. ABCB1 polymorphism as prognostic factor in breast cancer patients treated with docetaxel and doxorubicin neoadjuvant chemotherapy. Cancer Sci. 2015;106:86–93. 5. Lal S, Wong ZW, Sandanaraj E, Xiang X, Ang PCS, Lee EJD, et al. Influence of ABCB1 and ABCG2 polymorphisms on doxorubicin disposition in Asian breast cancer patients. Cancer Sci. 2008;99:816–23. 6. Voon PJ, Yap HL, Ma C-Y-T, Lu F, Wong ALA, Sapari NS, et al. Correlation of aldo-ketoreductase (AKR) 1C3 genetic variant with doxorubicin pharmacodynamics in Asian breast cancer patients. Br J Clin Pharmacol. 2013;75:1497–505. 7. Fukudo M, Ikemi Y, Togashi Y, Masago K, Kim YH, Mio T, et al. Population pharmacokinetics/pharmacodynamics of erlotinib and pharmacogenomic analysis of plasma and cerebrospinal fluid drug concentrations in Japanese patients with non-small cell lung cancer. Clin Pharmacokinet. 2013;52:593–609. 8. Gurney H, Wong M, Balleine RL, Rivory LP, McLachlan AJ, Hoskins JM, et al. Imatinib disposition and ABCB1 (MDR1, P-glycoprotein) genotype. Clin Pharmacol Ther. 2007;82:33–40. 9. Han J-Y, Lim H-S, Yoo Y-K, Shin ES, Park YH, Lee SY, et al. Associations of ABCB1, ABCC2, and ABCG2 polymorphisms with irinotecan-pharmacokinetics and clinical outcome in patients with advanced nonsmall cell lung cancer. Cancer. 2007;110:138–47. 10. Zhou Q, Sparreboom A, Tan E-H, Cheung Y-B, Lee A, Poon D, et al. Pharmacogenetic profiling across the irinotecan pathway in Asian patients with cancer. Br J Clin Pharmacol. 2005;59:415–24. 11. De Graan A-JM, Elens L, Sprowl JA, Sparreboom A, Friberg LE, van der Holt B, et al. CYP3A4*22 genotype and systemic exposure affect paclitaxel-induced neurotoxicity. Clin Cancer Res. 2013;19:3316–24. 12. Fransson MN, Gréen H, Litton J-E, Friberg LE. Influence of Cremophor EL and genetic polymorphisms on the pharmacokinetics of paclitaxel and its metabolites using a mechanism-based model. Drug Metab Dispos. 2011;39:247–55. 13. Gréen H, Söderkvist P, Rosenberg P, Mirghani RA, Rymark P, Lundqvist EA, et al. Pharmacogenetic studies of Paclitaxel in the treatment of ovarian cancer. Basic Clin Pharmacol Toxicol. 2009;104:130–7. 14. Jiko M, Yano I, Sato E, Takahashi K, Motohashi H, Masuda S, et al. Pharmacokinetics and pharmacodynamics of paclitaxel with carboplatin or gemcitabine, and effects of CYP3A5 and MDR1 polymorphisms in patients with urogenital cancers. Int J Clin Oncol. 2007;12:284–90. 15. Nakajima M, Fujiki Y, Kyo S, Kanaya T, Nakamura M, Maida Y, et al. Pharmacokinetics of paclitaxel in ovarian cancer patients and genetic polymorphisms of CYP2C8, CYP3A4, and MDR1. J Clin Pharmacol. 2005;45:674–82. 16. Sissung TM, Mross K, Steinberg SM, Behringer D, Figg WD, Sparreboom A, et al. Association of ABCB1 genotypes with paclitaxel-mediated peripheral neuropathy and neutropenia. Eur J Cancer. 2006;42:2893–6. 19 17. Mizuno T, Fukudo M, Terada T, Kamba T, Nakamura E, Ogawa O, et al. Impact of genetic variation in breast cancer resistance protein (BCRP/ABCG2) on sunitinib pharmacokinetics. Drug Metab Pharmacokinet. 2012;27:631–9. 18. Guilhaumou R, Solas C, Bourgarel-Rey V, Quaranta S, Rome A, Simon N, et al. Impact of plasma and intracellular exposure and CYP3A4, CYP3A5, and ABCB1 genetic polymorphisms on vincristine-induced neurotoxicity. Cancer Chemother Pharmacol. 2011;68:1633–8. 19. Kim K-A, Cha Y-J, Lee H-M, Joo H-J, Park J-Y. Effects of the ABCG2 and ABCB1 drug transporter polymorphisms on the pharmacokinetics of bicalutamide in humans. Clin Chim Acta. 2015;438:7–11. 20. Meng H, Guo G, Ren J, Zhou H, Ge Y, Guo Y. Effects of ABCB1 polymorphisms on plasma carbamazepine concentrations and pharmacoresistance in Chinese patients with epilepsy. Epilepsy Behav. 2011;21:27–30. 21. Ozgon GO, Bebek N, Gul G, Cine N. Association of MDR1 (C3435T) polymorphism and resistance to carbamazepine in epileptic patients from Turkey. Eur Neurol. 2008;59:67–70. 22. Puranik YG, Birnbaum AK, Marino SE, Ahmed G, Cloyd JC, Remmel RP, et al. Association of carbamazepine major metabolism and transport pathway gene polymorphisms and pharmacokinetics in patients with epilepsy. Pharmacogenomics. 2013;14:35–45. 23. Seo T, Ishitsu T, Ueda N, Nakada N, Yurube K, Ueda K, et al. ABCB1 polymorphisms influence the response to antiepileptic drugs in Japanese epilepsy patients. Pharmacogenomics. 2006;7:551–61. 24. Zhu X, Yun W, Sun X, Qiu F, Zhao L, Guo Y. Effects of major transporter and metabolizing enzyme gene polymorphisms on carbamazepine metabolism in Chinese patients with epilepsy. Pharmacogenomics. 2014;15:1867–79. 25. Kang H-A, Cho H-Y, Lee Y-B. The effect of MDR1 G2677T/A polymorphism on pharmacokinetics of gabapentin in healthy Korean subjects. Arch Pharm Res. 2007;30:96–101. 26. Lovrić M, Božina N, Hajnšek S, Kuzman MR, Sporiš D, Lalić Z, et al. Association between lamotrigine concentrations and ABCB1 polymorphisms in patients with epilepsy. Ther Drug Monit. 2012;34:518–25. 27. Basic S, Hajnsek S, Bozina N, Filipcic I, Sporis D, Mislov D, et al. The influence of C3435T polymorphism of ABCB1 gene on penetration of phenobarbital across the blood-brain barrier in patients with generalized epilepsy. Seizure. 2008;17:524–30. 28. Ponnala S, Chaudhari JR, Jaleel MA, Bhiladvala D, Kaipa PR, Das UN, et al. Role of MDR1 C3435T and GABRG2 C588T gene polymorphisms in seizure occurrence and MDR1 effect on anti-epileptic drug (phenytoin) absorption. Genet Test Mol Biomarkers. 2012;16:550–7. 29. Turgut G, Kurt E, Sengul C, Alatas G, Kursunluoglu R, Oral T, et al. Association of MDR1 C3435T polymorphism with bipolar disorder in patients treated with valproic acid. Mol Biol Rep. 2009;36:495–9. 30. Rodríguez Nóvoa S, Barreiro P, Rendón A, Barrios A, Corral A, Jiménez-Nacher I, et al. Plasma levels of atazanavir and the risk of hyperbilirubinemia are predicted by the 3435C-->T polymorphism at the multidrug resistance gene 1. Clin Infect Dis. 2006;42:291–5. 31. Rodríguez-Nóvoa S, Martín-Carbonero L, Barreiro P, González-Pardo G, Jiménez-Nácher I, González-Lahoz J, et al. Genetic factors influencing atazanavir plasma concentrations and the risk of severe hyperbilirubinemia. AIDS. 2007;21:41–6. 32. D’Avolio A, Carcieri C, Cusato J, Simiele M, Calcagno A, Allegra S, et al. Intracellular accumulation of atazanavir/ritonavir according to plasma concentrations and OATP1B1, ABCB1 and PXR genetic polymorphisms. J Antimicrob Chemother. 2014;69:3061–6. 33. He X-J, Zhao L-M, Qiu F, Sun Y-X, Li-Ling J. Influence of ABCB1 gene polymorphisms on the pharmacokinetics of azithromycin among healthy Chinese Han ethnic subjects. Pharmacol Rep. 2009;61:843–50. 20 34. Yin OQP, Tomlinson B, Chow MSS. Effect of multidrug resistance gene-1 (ABCB1) polymorphisms on the single-dose pharmacokinetics of cloxacillin in healthy adult Chinese men. Clin Ther. 2009;31:999–1006. 35. Beringer PM, Kriengkauykiat J, Zhang X, Hidayat L, Liu S, Louie S, et al. Lack of effect of P-glycoprotein inhibition on renal clearance of dicloxacillin in patients with cystic fibrosis. Pharmacotherapy. 2008;28:883–94. 36. Putnam WS, Woo JM, Huang Y, Benet LZ. Effect of the MDR1 C3435T variant and P-glycoprotein induction on dicloxacillin pharmacokinetics. J Clin Pharmacol. 2005;45:411–21. 37. Elens L, Vandercam B, Yombi J-C, Lison D, Wallemacq P, Haufroid V. Influence of host genetic factors on efavirenz plasma and intracellular pharmacokinetics in HIV-1-infected patients. Pharmacogenomics. 2010;11:1223–34. 38. Mukonzo JK, Röshammar D, Waako P, Andersson M, Fukasawa T, Milani L, et al. A novel polymorphism in ABCB1 gene, CYP2B6*6 and sex predict single-dose efavirenz population pharmacokinetics in Ugandans. British Journal of Clinical Pharmacology. 2009;68:690–9. 39. Mukonzo JK, Owen JS, Ogwal-Okeng J, Kuteesa RB, Nanzigu S, Sewankambo N, et al. Pharmacogeneticbased efavirenz dose modification: suggestions for an African population and the different CYP2B6 genotypes. PLoS ONE. 2014;9:e86919. 40. Ngaimisi E, Habtewold A, Minzi O, Makonnen E, Mugusi S, Amogne W, et al. Importance of ethnicity, CYP2B6 and ABCB1 genotype for efavirenz pharmacokinetics and treatment outcomes: a parallel-group prospective cohort study in two sub-Saharan Africa populations. PLoS ONE. 2013;8:e67946. 41. Winzer R, Langmann P, Zilly M, Tollmann F, Schubert J, Klinker H, et al. No influence of the Pglycoprotein genotype (MDR1 C3435T) on plasma levels of lopinavir and efavirenz during antiretroviral treatment. Eur J Med Res. 2003;8:531–4. 42. Fellay J, Marzolini C, Meaden ER, Back DJ, Buclin T, Chave JP, et al. Response to antiretroviral treatment in HIV-1-infected individuals with allelic variants of the multidrug resistance transporter 1: a pharmacogenetics study. Lancet. 2002;359:30–6. 43. Curras V, Hocht C, Mangano A, Niselman V, Mariño Hernández E, Cáceres Guido P, et al. Pharmacokinetic study of the variability of indinavir drug levels when boosted with ritonavir in HIV-infected children. Pharmacology. 2009;83:59–66. 44. Solas C, Simon N, Drogoul M-P, Quaranta S, Frixon-Marin V, Bourgarel-Rey V, et al. Minimal effect of MDR1 and CYP3A5 genetic polymorphisms on the pharmacokinetics of indinavir in HIV-infected patients. Br J Clin Pharmacol. 2007;64:353–62. 45. Ma Q, Brazeau D, Zingman BS, Reichman RC, Fischl MA, Gripshover BM, et al. Multidrug resistance 1 polymorphisms and trough concentrations of atazanavir and lopinavir in patients with HIV. Pharmacogenomics. 2007;8:227–35. 46. Estrela R de C, Ribeiro FS, Barroso PF, Tuyama M, Gregório SP, Dias-Neto E, et al. ABCB1 polymorphisms and the concentrations of lopinavir and ritonavir in blood, semen and saliva of HIV-infected men under antiretroviral therapy. Pharmacogenomics. 2009;10:311–8. 47. Colombo S, Soranzo N, Rotger M, Sprenger R, Bleiber G, Furrer H, et al. Influence of ABCB1, ABCC1, ABCC2, and ABCG2 haplotypes on the cellular exposure of nelfinavir in vivo. Pharmacogenet Genomics. 2005;15:599–608. 48. Saitoh A, Capparelli E, Aweeka F, Sarles E, Singh KK, Kovacs A, et al. CYP2C19 genetic variants affect nelfinavir pharmacokinetics and virologic response in HIV-1-infected children receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;54:285–9. 49. Fröhlich M, Burhenne J, Martin-Facklam M, Weiss J, von Wolff M, Strowitzki T, et al. Oral contraception does not alter single dose saquinavir pharmacokinetics in women. Br J Clin Pharmacol. 2004;57:244–52. 21 50. Zhang Y, Jiang X-H, Hu Y-Q, Li Z-R, Su L, Wang Z-G, et al. MDR1 genotypes do not influence the absorption of a single oral dose of 600 mg valacyclovir in healthy Chinese Han ethnic males. Br J Clin Pharmacol. 2008;66:247–54. 51. Consoli G, Lastella M, Ciapparelli A, Catena Dell’Osso M, Ciofi L, Guidotti E, et al. ABCB1 polymorphisms are associated with clozapine plasma levels in psychotic patients. Pharmacogenomics. 2009;10:1267–76. 52. Ghotbi R, Mannheimer B, Aklillu E, Suda A, Bertilsson L, Eliasson E, et al. Carriers of the UGT1A4 142T>G gene variant are predisposed to reduced olanzapine exposure--an impact similar to male gender or smoking in schizophrenic patients. Eur J Clin Pharmacol. 2010;66:465–74. 53. Cho HY, Yoo HD, Lee YB. Influence of ABCB1 genetic polymorphisms on the pharmacokinetics of levosulpiride in healthy subjects. Neuroscience. 2010;169:378–87. 54. Gunes A, Spina E, Dahl M-L, Scordo MG. ABCB1 polymorphisms influence steady-state plasma levels of 9hydroxyrisperidone and risperidone active moiety. Ther Drug Monit. 2008;30:628–33. 55. Jovanović N, Božina N, Lovrić M, Medved V, Jakovljević M, Peleš AM. The role of CYP2D6 and ABCB1 pharmacogenetics in drug-naïve patients with first-episode schizophrenia treated with risperidone. Eur J Clin Pharmacol. 2010;66:1109–17. 56. Kastelic M, Koprivsek J, Plesnicar BK, Serretti A, Mandelli L, Locatelli I, et al. MDR1 gene polymorphisms and response to acute risperidone treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:387–92. 57. Xiang Q, Zhao X, Zhou Y, Duan JL, Cui YM. Effect of CYP2D6, CYP3A5, and MDR1 Genetic Polymorphisms on the Pharmacokinetics of Risperidone and Its Active Moiety. The Journal of Clinical Pharmacology. 2010;50:659–66. 58. Yasui-Furukori N, Mihara K, Takahata T, Suzuki A, Nakagami T, De Vries R, et al. Effects of various factors on steady-state plasma concentrations of risperidone and 9-hydroxyrisperidone: lack of impact of MDR-1 genotypes. Br J Clin Pharmacol. 2004;57:569–75. 59. Bakken GV, Molden E, Hermann M. Impact of Genetic Variability in CYP2D6, CYP3A5, and ABCB1 on Serum Concentrations of Quetiapine and N-desalkylquetiapine in Psychiatric Patients. Ther Drug Monit. 2015;37:256–61. 60. Kim K-A, Park P-W, Park J-Y. Effect of ABCB1 (MDR1) haplotypes derived from G2677T/C3435T on the pharmacokinetics of amlodipine in healthy subjects. Br J Clin Pharmacol. 2007;63:53–8. 61. Zuo X-C, Zhang W-L, Yuan H, Barrett JS, Hua Y, Huang Z-J, et al. ABCB1 Polymorphism and Gender Affect the Pharmacokinetics of Amlodipine in Chinese Patients with Essential Hypertension: A Population Analysis. Drug Metab Pharmacokinet. 2014;29:305–11. 62. Aarnoudse A-JLHJ, Dieleman JP, Visser LE, Arp PP, van der Heiden IP, van Schaik RHN, et al. Common ATP-binding cassette B1 variants are associated with increased digoxin serum concentration. Pharmacogenet Genomics. 2008;18:299–305. 63. Gerloff T, Schaefer M, Johne A, Oselin K, Meisel C, Cascorbi I, et al. MDR1 genotypes do not influence the absorption of a single oral dose of 1 mg digoxin in healthy white males. Br J Clin Pharmacol. 2002;54:610–6. 64. Johne A, Köpke K, Gerloff T, Mai I, Rietbrock S, Meisel C, et al. Modulation of steady-state kinetics of digoxin by haplotypes of the P-glycoprotein MDR1 gene. Clin Pharmacol Ther. 2002;72:584–94. 65. Kurata Y, Ieiri I, Kimura M, Morita T, Irie S, Urae A, et al. Role of human MDR1 gene polymorphism in bioavailability and interaction of digoxin, a substrate of P-glycoprotein. Clin Pharmacol Ther. 2002;72:209–19. 66. Verstuyft C, Schwab M, Schaeffeler E, Kerb R, Brinkmann U, Jaillon P, et al. Digoxin pharmacokinetics and MDR1 genetic polymorphisms. Eur J Clin Pharmacol. 2003;58:809–12. 22 67. Verstuyft C, Strabach S, El-Morabet H, Kerb R, Brinkmann U, Dubert L, et al. Dipyridamole enhances digoxin bioavailability via P-glycoprotein inhibition. Clin Pharmacol Ther. 2003;73:51–60. 68. Xu P, Jiang Z-P, Zhang B-K, Tu J-Y, Li H-D. Impact of MDR1 haplotypes derived from C1236T, G2677T/A and C3435T on the pharmacokinetics of single-dose oral digoxin in healthy Chinese volunteers. Pharmacology. 2008;82:221–7. 69. Yasar U, Babaoglu MO, Bozkurt A. Disposition of a CYP2C9 phenotyping agent, losartan, is not influenced by the common 3435C > T variation of the drug transporter gene ABCB1 (MDR1). Basic Clin Pharmacol Toxicol. 2008;103:176–9. 70. Guo X, Chen X-P, Cheng Z-N, Luo X, Guo R, Chen L, et al. No effect of MDR1 C3435T polymorphism on oral pharmacokinetics of telmisartan in 19 healthy Chinese male subjects. Clin Chem Lab Med. 2009;47:38–43. 71. Pan W, Ryu JY, Shon JH, Song IS, Liu KH, Sunwoo YE, et al. Dietary salt does not influence the disposition of verapamil enantiomers in relation to efflux transporter ABCB1 genetic polymorphism in healthy Korean subjects. Xenobiotica. 2008;38:422–34. 72. Zhao L-M, He X-J, Qiu F, Sun Y-X, Li-Ling J. Influence of ABCB1 gene polymorphisms on the pharmacokinetics of verapamil among healthy Chinese Han ethnic subjects. Br J Clin Pharmacol. 2009;68:395– 401. 73. Anglicheau D, Verstuyft C, Laurent-Puig P, Becquemont L, Schlageter M-H, Cassinat B, et al. Association of the multidrug resistance-1 gene single-nucleotide polymorphisms with the tacrolimus dose requirements in renal transplant recipients. J Am Soc Nephrol. 2003;14:1889–96. 74. Bonhomme-Faivre L, Picard V, Saliba F, Abbara C, Fodil M, Chaunoy M, et al. Effect of the ABCB1 3435C>T polymorphism on tacrolimus concentrations and dosage requirements in liver transplant recipients. Am J Health Syst Pharm. 2009;66:1645–51. 75. Cheung CY, Op den Buijsch RAM, Wong KM, Chan HW, Chau KF, Li CS, et al. Influence of different allelic variants of the CYP3A and ABCB1 genes on the tacrolimus pharmacokinetic profile of Chinese renal transplant recipients. Pharmacogenomics. 2006;7:563–74. 76. Cho J-H, Yoon Y-D, Park J-Y, Song E-J, Choi J-Y, Yoon S-H, et al. Impact of cytochrome P450 3A and ATP-binding cassette subfamily B member 1 polymorphisms on tacrolimus dose-adjusted trough concentrations among Korean renal transplant recipients. Transplant Proc. 2012;44:109–14. 77. Choi JH, Lee YJ, Jang SB, Lee J-E, Kim KH, Park K. Influence of the CYP3A5 and MDR1 genetic polymorphisms on the pharmacokinetics of tacrolimus in healthy Korean subjects. Br J Clin Pharmacol. 2007;64:185–91. 78. Díaz-Molina B, Tavira B, Lambert JL, Bernardo MJ, Alvarez V, Coto E. Effect of CYP3A5, CYP3A4, and ABCB1 genotypes as determinants of tacrolimus dose and clinical outcomes after heart transplantation. Transplant Proc. 2012;44:2635–8. 79. Elens L, Capron A, Kerckhove VV, Lerut J, Mourad M, Lison D, et al. 1199G>A and 2677G>T/A polymorphisms of ABCB1 independently affect tacrolimus concentration in hepatic tissue after liver transplantation. Pharmacogenet Genomics. 2007;17:873–83. 80. Fredericks S, Moreton M, Reboux S, Carter ND, Goldberg L, Holt DW, et al. Multidrug resistance gene-1 (MDR-1) haplotypes have a minor influence on tacrolimus dose requirements. Transplantation. 2006;82:705–8. 81. Gervasini G, Garcia M, Macias RM, Cubero JJ, Caravaca F, Benitez J. Impact of genetic polymorphisms on tacrolimus pharmacokinetics and the clinical outcome of renal transplantation. Transpl Int. 2012;25:471–80. 82. Gómez-Bravo MA, Salcedo M, Fondevila C, Suarez F, Castellote J, Rufian S, et al. Impact of donor and recipient CYP3A5 and ABCB1 genetic polymorphisms on tacrolimus dosage requirements and rejection in Caucasian Spanish liver transplant patients. J Clin Pharmacol. 2013;53:1146–54. 23 83. Goto M, Masuda S, Saito H, Uemoto S, Kiuchi T, Tanaka K, et al. C3435T polymorphism in the MDR1 gene affects the enterocyte expression level of CYP3A4 rather than Pgp in recipients of living-donor liver transplantation. Pharmacogenetics. 2002;12:451–7. 84. Goto M, Masuda S, Kiuchi T, Ogura Y, Oike F, Okuda M, et al. CYP3A5*1-carrying graft liver reduces the concentration/oral dose ratio of tacrolimus in recipients of living-donor liver transplantation. Pharmacogenetics. 2004;14:471–8. 85. Haufroid V, Wallemacq P, VanKerckhove V, Elens L, De Meyer M, Eddour DC, et al. CYP3A5 and ABCB1 polymorphisms and tacrolimus pharmacokinetics in renal transplant candidates: guidelines from an experimental study. Am J Transplant. 2006;6:2706–13. 86. Hawwa AF, McKiernan PJ, Shields M, Millership JS, Collier PS, McElnay JC. Influence of ABCB1 polymorphisms and haplotypes on tacrolimus nephrotoxicity and dosage requirements in children with liver transplant. Br J Clin Pharmacol. 2009;68:413–21. 87. Hesselink DA, van Schaik RHN, van Agteren M, de Fijter JW, Hartmann A, Zeier M, et al. CYP3A5 genotype is not associated with a higher risk of acute rejection in tacrolimus-treated renal transplant recipients. Pharmacogenet Genomics. 2008;18:339–48. 88. Hosohata K, Masuda S, Yonezawa A, Katsura T, Oike F, Ogura Y, et al. MDR1 haplotypes conferring an increased expression of intestinal CYP3A4 rather than MDR1 in female living-donor liver transplant patients. Pharm Res. 2009;26:1590–5. 89. Jun KR, Lee W, Jang MS, Chun S, Song G-W, Park KT, et al. Tacrolimus concentrations in relation to CYP3A and ABCB1 polymorphisms among solid organ transplant recipients in Korea. Transplantation. 2009;87:1225–31. 90. Kuypers DRJ, de Jonge H, Naesens M, Lerut E, Verbeke K, Vanrenterghem Y. CYP3A5 and CYP3A4 but not MDR1 single-nucleotide polymorphisms determine long-term tacrolimus disposition and drug-related nephrotoxicity in renal recipients. Clin Pharmacol Ther. 2007;82:711–25. 91. Kuypers DRJ, Naesens M, de Jonge H, Lerut E, Verbeke K, Vanrenterghem Y. Tacrolimus dose requirements and CYP3A5 genotype and the development of calcineurin inhibitor-associated nephrotoxicity in renal allograft recipients. Ther Drug Monit. 2010;32:394–404. 92. Li D, Zhu J-Y, Gao J, Wang X, Lou Y-Q, Zhang G-L. Polymorphisms of tumor necrosis factor-alpha, interleukin-10, cytochrome P450 3A5 and ABCB1 in Chinese liver transplant patients treated with immunosuppressant tacrolimus. Clin Chim Acta. 2007;383:133–9. 93. Li D, Lu W, Zhu J-Y, Gao J, Lou Y-Q, Zhang G-L. Population pharmacokinetics of tacrolimus and CYP3A5, MDR1 and IL-10 polymorphisms in adult liver transplant patients. J Clin Pharm Ther. 2007;32:505– 15. 94. López-Montenegro Soria MA, Kanter Berga J, Beltrán Catalán S, Milara Payá J, Pallardó Mateu LM, Jiménez Torres NV. Genetic polymorphisms and individualized tacrolimus dosing. Transplant Proc. 2010;42:3031–3. 95. Macphee IAM, Fredericks S, Tai T, Syrris P, Carter ND, Johnston A, et al. Tacrolimus pharmacogenetics: polymorphisms associated with expression of cytochrome p4503A5 and P-glycoprotein correlate with dose requirement. Transplantation. 2002;74:1486–9. 96. MacPhee IAM, Fredericks S, Tai T, Syrris P, Carter ND, Johnston A, et al. The influence of pharmacogenetics on the time to achieve target tacrolimus concentrations after kidney transplantation. Am J Transplant. 2004;4:914–9. 97. Mai I, Perloff ES, Bauer S, Goldammer M, Johne A, Filler G, et al. MDR1 haplotypes derived from exons 21 and 26 do not affect the steady-state pharmacokinetics of tacrolimus in renal transplant patients. Br J Clin Pharmacol. 2004;58:548–53. 24 98. Min S-I, Kim SY, Ahn SH, Min S-K, Kim SH, Kim YS, et al. CYP3A5 *1 allele: impacts on early acute rejection and graft function in tacrolimus-based renal transplant recipients. Transplantation. 2010;90:1394–400. 99. Mourad M, Wallemacq P, De Meyer M, Brandt D, Van Kerkhove V, Malaise J, et al. The influence of genetic polymorphisms of cytochrome P450 3A5 and ABCB1 on starting dose- and weight-standardized tacrolimus trough concentrations after kidney transplantation in relation to renal function. Clin Chem Lab Med. 2006;44:1192–8. 100. Op den Buijsch RAM, Christiaans MHL, Stolk LML, de Vries JE, Cheung CY, Undre NA, et al. Tacrolimus pharmacokinetics and pharmacogenetics: influence of adenosine triphosphate-binding cassette B1 (ABCB1) and cytochrome (CYP) 3A polymorphisms. Fundam Clin Pharmacol. 2007;21:427–35. 101. Provenzani A, Notarbartolo M, Labbozzetta M, Poma P, Biondi F, Sanguedolce R, et al. The effect of CYP3A5 and ABCB1 single nucleotide polymorphisms on tacrolimus dose requirements in Caucasian liver transplant patients. Ann Transplant. 2009;14:23–31. 102. Provenzani A, Notarbartolo M, Labbozzetta M, Poma P, Vizzini G, Salis P, et al. Influence of CYP3A5 and ABCB1 gene polymorphisms and other factors on tacrolimus dosing in Caucasian liver and kidney transplant patients. Int J Mol Med. 2011;28:1093–102. 103. Roy JN, Barama A, Poirier C, Vinet B, Roger M. Cyp3A4, Cyp3A5, and MDR-1 genetic influences on tacrolimus pharmacokinetics in renal transplant recipients. Pharmacogenet Genomics. 2006;16:659–65. 104. Shi Y, Li Y, Tang J, Zhang J, Zou Y, Cai B, et al. Influence of CYP3A4, CYP3A5 and MDR-1 polymorphisms on tacrolimus pharmacokinetics and early renal dysfunction in liver transplant recipients. Gene. 2013;512:226–31. 105. Shilbayeh S. The impact of genetic polymorphisms on time required to attain the target tacrolimus levels and subsequent pharmacodynamic outcomes in pediatric kidney transplant patients. Saudi J Kidney Dis Transpl. 2014;25:266–77. 106. Tada H, Tsuchiya N, Satoh S, Kagaya H, Li Z, Sato K, et al. Impact of CYP3A5 and MDR1(ABCB1) C3435T polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipients. Transplant Proc. 2005;37:1730–2. 107. Tsuchiya N, Satoh S, Tada H, Li Z, Ohyama C, Sato K, et al. Influence of CYP3A5 and MDR1 (ABCB1) polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipients. Transplantation. 2004;78:1182–7. 108. Wang J, Zeevi A, McCurry K, Schuetz E, Zheng H, Iacono A, et al. Impact of ABCB1 (MDR1) haplotypes on tacrolimus dosing in adult lung transplant patients who are CYP3A5 *3/*3 non-expressors. Transpl Immunol. 2006;15:235–40. 109. Wei-lin W, Jing J, Shu-sen Z, Li-hua Wu, Ting-bo L, Song-feng Y, et al. Tacrolimus dose requirement in relation to donor and recipient ABCB1 and CYP3A5 gene polymorphisms in Chinese liver transplant patients. Liver Transpl. 2006;12:775–80. 110. Zhang X, Liu Z, Zheng J, Chen Z, Tang Z, Chen J, et al. Influence of CYP3A5 and MDR1 polymorphisms on tacrolimus concentration in the early stage after renal transplantation. Clin Transplant. 2005;19:638–43. 111. Zheng H, Zeevi A, Schuetz E, Lamba J, McCurry K, Griffith BP, et al. Tacrolimus dosing in adult lung transplant patients is related to cytochrome P4503A5 gene polymorphism. J Clin Pharmacol. 2004;44:135–40. 112. Mourad M, Mourad G, Wallemacq P, Garrigue V, Van Bellingen C, Van Kerckhove V, et al. Sirolimus and tacrolimus trough concentrations and dose requirements after kidney transplantation in relation to CYP3A5 and MDR1 polymorphisms and steroids. Transplantation. 2005;80:977–84. 113. Renders L, Frisman M, Ufer M, Mosyagin I, Haenisch S, Ott U, et al. CYP3A5 genotype markedly influences the pharmacokinetics of tacrolimus and sirolimus in kidney transplant recipients. Clin Pharmacol Ther. 2007;81:228–34. 25 114. Lemaitre F, Bezian E, Goldwirt L, Fernandez C, Farinotti R, Varnous S, et al. Population pharmacokinetics of everolimus in cardiac recipients: comedications, ABCB1, and CYP3A5 polymorphisms. Ther Drug Monit. 2012;34:686–94. 115. Bandur S, Petrasek J, Hribova P, Novotna E, Brabcova I, Viklicky O. Haplotypic structure of ABCB1/MDR1 gene modifies the risk of the acute allograft rejection in renal transplant recipients. Transplantation. 2008;86:1206–13. 116. Haufroid V, Mourad M, Van Kerckhove V, Wawrzyniak J, De Meyer M, Eddour DC, et al. The effect of CYP3A5 and MDR1 (ABCB1) polymorphisms on cyclosporine and tacrolimus dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenetics. 2004;14:147–54. 117. Hesselink DA, van Schaik RHN, van der Heiden IP, van der Werf M, Gregoor PJHS, Lindemans J, et al. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther. 2003;74:245–54. 118. Loh PT, Lou HX, Zhao Y, Chin YM, Vathsala A. Significant impact of gene polymorphisms on tacrolimus but not cyclosporine dosing in Asian renal transplant recipients. Transplant Proc. 2008;40:1690–5. 119. Onizuka M, Kunii N, Toyosaki M, Machida S, Ohgiya D, Ogawa Y, et al. Cytochrome P450 genetic polymorphisms influence the serum concentration of calcineurin inhibitors in allogeneic hematopoietic SCT recipients. Bone Marrow Transplant. 2011;46:1113–7. 120. Singh R, Srivastava A, Kapoor R, Mittal RD. Do drug transporter (ABCB1) SNPs influence cyclosporine and tacrolimus dose requirements and renal allograft outcome in the posttransplantation period? J Clin Pharmacol. 2011;51:603–15. 121. Anglicheau D, Thervet E, Etienne I, Hurault De Ligny B, Le Meur Y, Touchard G, et al. CYP3A5 and MDR1 genetic polymorphisms and cyclosporine pharmacokinetics after renal transplantation. Clin Pharmacol Ther. 2004;75:422–33. 122. Azarpira N, Aghdaie MH, Behzad-Behbahanie A, Geramizadeh B, Behzadi S, Malekhoseinie SA, et al. Association between cyclosporine concentration and genetic polymorphisms of CYP3A5 and MDR1 during the early stage after renal transplantation. Exp Clin Transplant. 2006;4:416–9. 123. Bonhomme-Faivre L, Devocelle A, Saliba F, Chatled S, Maccario J, Farinotti R, et al. MDR-1 C3435T polymorphism influences cyclosporine a dose requirement in liver-transplant recipients. Transplantation. 2004;78:21–5. 124. Bouamar R, Hesselink DA, van Schaik RHN, Weimar W, Macphee IAM, de Fijter JW, et al. Polymorphisms in CYP3A5, CYP3A4, and ABCB1 are not associated with cyclosporine pharmacokinetics nor with cyclosporine clinical end points after renal transplantation. Ther Drug Monit. 2011;33:178–84. 125. Chowbay B, Cumaraswamy S, Cheung YB, Zhou Q, Lee EJD. Genetic polymorphisms in MDR1 and CYP3A4 genes in Asians and the influence of MDR1 haplotypes on cyclosporin disposition in heart transplant recipients. Pharmacogenetics. 2003;13:89–95. 126. Crettol S, Venetz J-P, Fontana M, Aubert J-D, Ansermot N, Fathi M, et al. Influence of ABCB1 genetic polymorphisms on cyclosporine intracellular concentration in transplant recipients. Pharmacogenet Genomics. 2008;18:307–15. 127. Fanta S, Niemi M, Jönsson S, Karlsson MO, Holmberg C, Neuvonen PJ, et al. Pharmacogenetics of cyclosporine in children suggests an age-dependent influence of ABCB1 polymorphisms. Pharmacogenet Genomics. 2008;18:77–90. 128. Foote CJ, Greer W, Kiberd BA, Fraser A, Lawen J, Nashan B, et al. MDR1 C3435T polymorphisms correlate with cyclosporine levels in de novo renal recipients. Transplant Proc. 2006;38:2847–9. 129. García M, Macías RM, Cubero JJ, Benítez J, Caravaca F, Gervasini G. ABCB1 polymorphisms are associated with cyclosporine-induced nephrotoxicity and gingival hyperplasia in renal transplant recipients. Eur J Clin Pharmacol. 2013;69:385–93. 26 130. Hesselink DA, van Gelder T, van Schaik RHN, Balk AHMM, van der Heiden IP, van Dam T, et al. Population pharmacokinetics of cyclosporine in kidney and heart transplant recipients and the influence of ethnicity and genetic polymorphisms in the MDR-1, CYP3A4, and CYP3A5 genes. Clin Pharmacol Ther. 2004;76:545–56. 131. Hu Y-F, Qiu W, Liu Z-Q, Zhu L-J, Liu Z-Q, Tu J-H, et al. Effects of genetic polymorphisms of CYP3A4, CYP3A5 and MDR1 on cyclosporine pharmacokinetics after renal transplantation. Clin Exp Pharmacol Physiol. 2006;33:1093–8. 132. Kuzuya T, Kobayashi T, Moriyama N, Nagasaka T, Yokoyama I, Uchida K, et al. Amlodipine, but not MDR1 polymorphisms, alters the pharmacokinetics of cyclosporine A in Japanese kidney transplant recipients. Transplantation. 2003;76:865–8. 133. Mai I, Störmer E, Goldammer M, Johne A, Krüger H, Budde K, et al. MDR1 haplotypes do not affect the steady-state pharmacokinetics of cyclosporine in renal transplant patients. J Clin Pharmacol. 2003;43:1101–7. 134. Min DI, Ellingrod VL, Marsh S, McLeod H. CYP3A5 polymorphism and the ethnic differences in cyclosporine pharmacokinetics in healthy subjects. Ther Drug Monit. 2004;26:524–8. 135. Qiu X-Y, Jiao Z, Zhang M, Zhong L-J, Liang H-Q, Ma C-L, et al. Association of MDR1, CYP3A4*18B, and CYP3A5*3 polymorphisms with cyclosporine pharmacokinetics in Chinese renal transplant recipients. Eur J Clin Pharmacol. 2008;64:1069–84. 136. Taegtmeyer AB, Breen JB, Smith J, Burke M, Leaver N, Pantelidis P, et al. ATP-binding cassette subfamily B member 1 polymorphisms do not determine cyclosporin exposure, acute rejection or nephrotoxicity after heart transplantation. Transplantation. 2010;89:75–82. 137. Wang Y, Wang C, Li J, Wang X, Zhu G, Chen X, et al. Effect of genetic polymorphisms of CYP3A5 and MDR1 on cyclosporine concentration during the early stage after renal transplantation in Chinese patients cotreated with diltiazem. Eur J Clin Pharmacol. 2009;65:239–47. 138. Yates CR, Zhang W, Song P, Li S, Gaber AO, Kotb M, et al. The effect of CYP3A5 and MDR1 polymorphic expression on cyclosporine oral disposition in renal transplant patients. J Clin Pharmacol. 2003;43:555–64. 139. Kim I-W, Yun H, Choi B, Han N, Park S-Y, Lee ES, et al. ABCB1 C3435T genetic polymorphism on population pharmacokinetics of methotrexate after hematopoietic stem cell transplantation in Korean patients: a prospective analysis. Clin Ther. 2012;34:1816–26. 140. Dilger K, Cascorbi I, Grünhage F, Hohenester S, Sauerbruch T, Beuers U. Multidrug resistance 1 genotype and disposition of budesonide in early primary biliary cirrhosis. Liver Int. 2006;26:285–90. 141. Miura M, Satoh S, Inoue K, Kagaya H, Saito M, Inoue T, et al. Influence of CYP3A5, ABCB1 and NR1I2 polymorphisms on prednisolone pharmacokinetics in renal transplant recipients. Steroids. 2008;73:1052–9. 142. Frelinger AL, Bhatt DL, Lee RD, Mulford DJ, Wu J, Nudurupati S, et al. Clopidogrel pharmacokinetics and pharmacodynamics vary widely despite exclusion or control of polymorphisms (CYP2C19, ABCB1, PON1), noncompliance, diet, smoking, co-medications (including proton pump inhibitors), and pre-existent variability in platelet function. J Am Coll Cardiol. 2013;61:872–9. 143. Taubert D, von Beckerath N, Grimberg G, Lazar A, Jung N, Goeser T, et al. Impact of P-glycoprotein on clopidogrel absorption. Clin Pharmacol Ther. 2006;80:486–501. 144. Karaźniewicz-Łada M, Danielak D, Rubiś B, Burchardt P, Komosa A, Lesiak M, et al. Impact of common ABCB1 polymorphism on pharmacokinetics and pharmacodynamics of clopidogrel and its metabolites. J Clin Pharm Ther. 2015;40:226–31. 145. Keskitalo JE, Kurkinen KJ, Neuvonen M, Backman JT, Neuvonen PJ, Niemi M. No significant effect of ABCB1 haplotypes on the pharmacokinetics of fluvastatin, pravastatin, lovastatin, and rosuvastatin. Br J Clin Pharmacol. 2009;68:207–13. 27 146. Drescher S, Schaeffeler E, Hitzl M, Hofmann U, Schwab M, Brinkmann U, et al. MDR1 gene polymorphisms and disposition of the P-glycoprotein substrate fexofenadine. Br J Clin Pharmacol. 2002;53:526– 34. 147. Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI, et al. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001;70:189– 99. 148. Kodaira C, Sugimoto M, Nishino M, Yamade M, Shirai N, Uchida S, et al. Effect of MDR1 C3435T polymorphism on lansoprazole in healthy Japanese subjects. Eur J Clin Pharmacol. 2009;65:593–600. 149. Pauli-Magnus C, Feiner J, Brett C, Lin E, Kroetz DL. No effect of MDR1 C3435T variant on loperamide disposition and central nervous system effects. Clin Pharmacol Ther. 2003;74:487–98. 150. Skarke C, Jarrar M, Schmidt H, Kauert G, Langer M, Geisslinger G, et al. Effects of ABCB1 (multidrug resistance transporter) gene mutations on disposition and central nervous effects of loperamide in healthy volunteers. Pharmacogenetics. 2003;13:651–60. 151. Cho H-Y, Yoon H, Park G-K, Lee Y-B. Pharmacokinetics and bioequivalence of two formulations of rebamipide 100-mg tablets: a randomized, single-dose, two-period, two-sequence crossover study in healthy Korean male volunteers. Clin Ther. 2009;31:2712–21. 152. Coller JK, Barratt DT, Dahlen K, Loennechen MH, Somogyi AA. ABCB1 genetic variability and methadone dosage requirements in opioid-dependent individuals. Clin Pharmacol Ther. 2006;80:682–90. 153. Lötsch J, Skarke C, Wieting J, Oertel BG, Schmidt H, Brockmöller J, et al. Modulation of the central nervous effects of levomethadone by genetic polymorphisms potentially affecting its metabolism, distribution, and drug action. Clin Pharmacol Ther. 2006;79:72–89. 154. Campa D, Gioia A, Tomei A, Poli P, Barale R. Association of ABCB1/MDR1 and OPRM1 gene polymorphisms with morphine pain relief. Clin Pharmacol Ther. 2008;83:559–66. 155. Coulbault L, Beaussier M, Verstuyft C, Weickmans H, Dubert L, Trégouet D, et al. Environmental and genetic factors associated with morphine response in the postoperative period. Clin Pharmacol Ther. 2006;79:316–24. 156. Fujita K, Ando Y, Yamamoto W, Miya T, Endo H, Sunakawa Y, et al. Association of UGT2B7 and ABCB1 genotypes with morphine-induced adverse drug reactions in Japanese patients with cancer. Cancer Chemother Pharmacol. 2010;65:251–8. 157. Lötsch J, von Hentig N, Freynhagen R, Griessinger N, Zimmermann M, Doehring A, et al. Cross-sectional analysis of the influence of currently known pharmacogenetic modulators on opioid therapy in outpatient pain centers. Pharmacogenet Genomics. 2009;19:429–36. 158. Ross JR, Riley J, Taegetmeyer AB, Sato H, Gretton S, du Bois RM, et al. Genetic variation and response to morphine in cancer patients: catechol-O-methyltransferase and multidrug resistance-1 gene polymorphisms are associated with central side effects. Cancer. 2008;112:1390–403. 159. Sia AT, Sng BL, Lim EC, Law H, Tan EC. The influence of ATP-binding cassette sub-family B member -1 (ABCB1) genetic polymorphisms on acute and chronic pain after intrathecal morphine for caesarean section: a prospective cohort study. Int J Obstet Anesth. 2010;19:254–60. 160. Zwisler ST, Enggaard TP, Noehr-Jensen L, Mikkelsen S, Verstuyft C, Becquemont L, et al. The antinociceptive effect and adverse drug reactions of oxycodone in human experimental pain in relation to genetic variations in the OPRM1 and ABCB1 genes. Fundam Clin Pharmacol. 2010;24:517–24. 161. Zwisler ST, Enggaard TP, Mikkelsen S, Verstuyft C, Becquemont L, Sindrup SH, et al. Lack of Association of OPRM1 and ABCB1 Single-Nucleotide Polymorphisms to Oxycodone Response in Postoperative Pain. J Clin Pharmacol. 2011; 28 162. Gonzalez-Haba E, García MI, Cortejoso L, López-Lillo C, Barrueco N, García-Alfonso P, et al. ABCB1 gene polymorphisms are associated with adverse reactions in fluoropyrimidine-treated colorectal cancer patients. Pharmacogenomics. 2010;11:1715–23. 163. Vulsteke C, Pfeil AM, Schwenkglenks M, Pettengell R, Szucs TD, Lambrechts D, et al. Impact of genetic variability and treatment-related factors on outcome in early breast cancer patients receiving (neo-) adjuvant chemotherapy with 5-fluorouracil, epirubicin and cyclophosphamide, and docetaxel. Breast Cancer Res Treat. 2014;147:557–70. 164. Paule B, Castagne V, Picard V, Saffroy R, Adam R, Guettier C, et al. MDR1 polymorphism role in patients treated with cetuximab and irinotecan in irinotecan refractory colorectal cancer. Med Oncol. 2010;27:1066–72. 165. Chen S, Huo X, Lin Y, Ban H, Lin Y, Li W, et al. Association of MDR1 and ERCC1 polymorphisms with response and toxicity to cisplatin-based chemotherapy in non-small-cell lung cancer patients. Int J Hyg Environ Health. 2010;213:140–5. 166. Sissung TM, Baum CE, Deeken J, Price DK, Aragon-Ching J, Steinberg SM, et al. ABCB1 genetic variation influences the toxicity and clinical outcome of patients with androgen-independent prostate cancer treated with docetaxel. Clin Cancer Res. 2008;14:4543–9. 167. Tsai S-M, Lin C-Y, Wu S-H, Hou LA, Ma H, Tsai L-Y, et al. Side effects after docetaxel treatment in Taiwanese breast cancer patients with CYP3A4, CYP3A5, and ABCB1 gene polymorphisms. Clin Chim Acta. 2009;404:160–5. 168. Choi JR, Kim J-O, Kang DR, Shin J-Y, Zhang XH, Oh JE, et al. Genetic Variations of Drug Transporters Can Influence on Drug Response in Patients Treated with Docetaxel Chemotherapy. Cancer Res Treat. 2014; 169. Sohn JW, Lee SY, Lee SJ, Kim EJ, Cha SI, Kim CH, et al. MDR1 polymorphisms predict the response to etoposide-cisplatin combination chemotherapy in small cell lung cancer. Jpn J Clin Oncol. 2006;36:137–41. 170. Kasuya K, Tsuchida A, Nagakawa Y, Suzuki Y, Suzuki M, Aoki T, et al. Prediction of a side effect and efficacy of adjuvant chemotherapy with gemcitabine for post operative patient of pancreatic cancer by a genetic polymorphism analysis. Hepatogastroenterology. 2012;59:1609–13. 171. Deenik W, van der Holt B, Janssen JJWM, Chu IWT, Valk PJM, Ossenkoppele GJ, et al. Polymorphisms in the multidrug resistance gene MDR1 (ABCB1) predict for molecular resistance in patients with newly diagnosed chronic myeloid leukemia receiving high-dose imatinib. Blood. 2010;116:6144–5; author reply 6145–6. 172. Dulucq S, Bouchet S, Turcq B, Lippert E, Etienne G, Reiffers J, et al. Multidrug resistance gene (MDR1) polymorphisms are associated with major molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood. 2008;112:2024–7. 173. Elghannam DM, Ibrahim L, Ebrahim MA, Azmy E, Hakem H. Association of MDR1 gene polymorphism (G2677T) with imatinib response in Egyptian chronic myeloid leukemia patients. Hematology. 2014;19:123–8. 174. Ni L-N, Li J-Y, Miao K-R, Qiao C, Zhang S-J, Qiu H-R, et al. Multidrug resistance gene (MDR1) polymorphisms correlate with imatinib response in chronic myeloid leukemia. Med Oncol. 2011;28:265–9. 175. Lara PN, Natale R, Crowley J, Lenz HJ, Redman MW, Carleton JE, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27:2530–5. 176. De Mattia E, Toffoli G, Polesel J, D’Andrea M, Corona G, Zagonel V, et al. Pharmacogenetics of ABC and SLC transporters in metastatic colorectal cancer patients receiving first-line FOLFIRI treatment. Pharmacogenet Genomics. 2013;23:549–57. 177. Chang H, Rha SY, Jeung H-C, Im C-K, Ahn JB, Kwon WS, et al. Association of the ABCB1 gene polymorphisms 2677G>T/A and 3435C>T with clinical outcomes of paclitaxel monotherapy in metastatic breast cancer patients. Ann Oncol. 2009;20:272–7. 29 178. Chang H, Rha SY, Jeung H-C, Im CK, Noh SH, Kim JJ, et al. Association of the ABCB1 3435C>T polymorphism and treatment outcomes in advanced gastric cancer patients treated with paclitaxel-based chemotherapy. Oncol Rep. 2010;23:271–8. 179. Hertz DL, Motsinger-Reif AA, Drobish A, Winham SJ, McLeod HL, Carey LA, et al. CYP2C8*3 predicts benefit/risk profile in breast cancer patients receiving neoadjuvant paclitaxel. Breast Cancer Res Treat. 2012;134:401–10. 180. Marsh S, Paul J, King CR, Gifford G, McLeod HL, Brown R. Pharmacogenetic assessment of toxicity and outcome after platinum plus taxane chemotherapy in ovarian cancer: the Scottish Randomised Trial in Ovarian Cancer. J Clin Oncol. 2007;25:4528–35. 181. Gréen H, Söderkvist P, Rosenberg P, Horvath G, Peterson C. ABCB1 G1199A polymorphism and ovarian cancer response to paclitaxel. J Pharm Sci. 2008;97:2045–8. 182. Gréen H, Söderkvist P, Rosenberg P, Horvath G, Peterson C. mdr-1 single nucleotide polymorphisms in ovarian cancer tissue: G2677T/A correlates with response to paclitaxel chemotherapy. Clin Cancer Res. 2006;12:854–9. 183. Beuselinck B, Karadimou A, Lambrechts D, Claes B, Wolter P, Couchy G, et al. Single-nucleotide polymorphisms associated with outcome in metastatic renal cell carcinoma treated with sunitinib. Br J Cancer. 2013;108:887–900. 184. Beuselinck B, Lambrechts D, Van Brussel T, Wolter P, Cardinaels N, Joniau S, et al. Efflux pump ABCB1 single nucleotide polymorphisms and dose reductions in patients with metastatic renal cell carcinoma treated with sunitinib. Acta Oncol. 2014;53:1413–22. 185. Garcia-Donas J, Esteban E, Leandro-García LJ, Castellano DE, del Alba AG, Climent MA, et al. Single nucleotide polymorphism associations with response and toxic effects in patients with advanced renal-cell carcinoma treated with first-line sunitinib: a multicentre, observational, prospective study. Lancet Oncol. 2011;12:1143–50. 186. Van Erp NP, Eechoute K, van der Veldt AA, Haanen JB, Reyners AKL, Mathijssen RHJ, et al. Pharmacogenetic pathway analysis for determination of sunitinib-induced toxicity. J Clin Oncol. 2009;27:4406– 12. 187. Schaich M, Kestel L, Pfirrmann M, Robel K, Illmer T, Kramer M, et al. A MDR1 (ABCB1) gene single nucleotide polymorphism predicts outcome of temozolomide treatment in glioblastoma patients. Ann Oncol. 2009;20:175–81. 188. Pan J, Han J, Wu J, Sheng L, Huang H, Yu Q. MDR1 single nucleotide polymorphisms predict response to vinorelbine-based chemotherapy in patients with non-small cell lung cancer. Respiration. 2008;75:380–5. 189. Zgheib NK, Akra-Ismail M, Aridi C, Mahfouz R, Abboud MR, Solh H, et al. Genetic polymorphisms in candidate genes predict increased toxicity with methotrexate therapy in Lebanese children with acute lymphoblastic leukemia. Pharmacogenet Genomics. 2014;24:387–96. 190. Gregers J, Gréen H, Christensen IJ, Dalhoff K, Schroeder H, Carlsen N, et al. Polymorphisms in the ABCB1 gene and effect on outcome and toxicity in childhood acute lymphoblastic leukemia. Pharmacogenomics J. 2015; 191. Uhr M, Tontsch A, Namendorf C, Ripke S, Lucae S, Ising M, et al. Polymorphisms in the drug transporter gene ABCB1 predict antidepressant treatment response in depression. Neuron. 2008;57:203–9. 192. Karlsson L, Green H, Zackrisson AL, Bengtsson F, Jakobsen Falk I, Carlsson B, et al. ABCB1 gene polymorphisms are associated with fatal intoxications involving venlafaxine but not citalopram. Int J Legal Med. 2013;127:579–86. 193. Ozbey G, Yucel B, Taycan SE, Kan D, Bodur NE, Arslan T, et al. ABCB1 C3435T polymorphism is associated with susceptibility to major depression, but not with a clinical response to citalopram in a Turkish population. Pharmacol Rep. 2014;66:235–8. 30 194. Lin K-M, Chiu Y-F, Tsai I-J, Chen C-H, Shen WW, Liu SC, et al. ABCB1 gene polymorphisms are associated with the severity of major depressive disorder and its response to escitalopram treatment: Pharmacogenetics and Genomics. 2010;1. 195. Kato M, Fukuda T, Serretti A, Wakeno M, Okugawa G, Ikenaga Y, et al. ABCB1 (MDR1) gene polymorphisms are associated with the clinical response to paroxetine in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:398–404. 196. Mihaljevic Peles A, Bozina N, Sagud M, Rojnic Kuzman M, Lovric M. MDR1 gene polymorphism: therapeutic response to paroxetine among patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1439–44. 197. Sarginson JE, Lazzeroni LC, Ryan HS, Ershoff BD, Schatzberg AF, Murphy GM. ABCB1 (MDR1) polymorphisms and antidepressant response in geriatric depression: Pharmacogenetics and Genomics. 2010;20:467–75. 198. Gassó P, Rodríguez N, Mas S, Pagerols M, Blázquez A, Plana MT, et al. Effect of CYP2D6, CYP2C9 and ABCB1 genotypes on fluoxetine plasma concentrations and clinical improvement in children and adolescent patients. Pharmacogenomics J. 2014;14:457–62. 199. Menu P, Gressier F, Verstuyft C, Hardy P, Becquemont L, Corruble E. Antidepressants and ABCB1 gene C3435T functional polymorphism: a naturalistic study. Neuropsychobiology. 2010;62:193–7. 200. Parkman HP, Jacobs MR, Mishra A, Hurdle JA, Sachdeva P, Gaughan JP, et al. Domperidone treatment for gastroparesis: demographic and pharmacogenetic characterization of clinical efficacy and side-effects. Dig Dis Sci. 2011;56:115–24. 201. Babaoglu MO, Bayar B, Aynacioglu AS, Kerb R, Abali H, Celik I, et al. Association of the ABCB1 3435C>T polymorphism with antiemetic efficacy of 5-hydroxytryptamine type 3 antagonists. Clin Pharmacol Ther. 2005;78:619–26. 202. Tsuji D, Kim Y-I, Nakamichi H, Daimon T, Suwa K, Iwabe Y, et al. Association of ABCB1 polymorphisms with the antiemetic efficacy of granisetron plus dexamethasone in breast cancer patients. Drug Metab Pharmacokinet. 2013;28:299–304. 203. Choi EM, Lee MG, Lee SH, Choi KW, Choi SH. Association of ABCB1 polymorphisms with the efficacy of ondansetron for postoperative nausea and vomiting. Anaesthesia. 2010;65:996–1000. 204. Perwitasari DA, Wessels JAM, van der Straaten RJHM, Baak-Pablo RF, Mustofa M, Hakimi M, et al. Association of ABCB1, 5-HT3B receptor and CYP2D6 genetic polymorphisms with ondansetron and metoclopramide antiemetic response in Indonesian cancer patients treated with highly emetogenic chemotherapy. Jpn J Clin Oncol. 2011;41:1168–76. 205. Zoto T, Kilickap S, Yasar U, Celik I, Bozkurt A, Babaoglu MO. Improved Anti-Emetic Efficacy of 5-HT3 Receptor Antagonists in Cancer Patients with Genetic Polymorphisms of ABCB1 (MDR1) Drug Transporter. Basic Clin Pharmacol Toxicol. 2015;116:354–60. 206. Subenthiran S, Abdullah NR, Muniandy PK, Joseph JP, Cheong KC, Ismail Z, et al. G2677T polymorphism can predict treatment outcome of Malaysians with complex partial seizures being treated with Carbamazepine. Genet Mol Res. 2013;12:5937–44. 207. Ebid A-HIM, Ahmed MMM, Mohammed SA. Therapeutic drug monitoring and clinical outcomes in epileptic Egyptian patients: a gene polymorphism perspective study. Ther Drug Monit. 2007;29:305–12. 208. Haerian BS, Lim KS, Tan HJ, Mohamed EHM, Tan CT, Raymond AA, et al. Association between ABCB1 polymorphism and response to sodium valproate treatment in Malaysian epilepsy patients. Epileptic Disord. 2011;13:65–75. 209. Alpman A, Ozkinay F, Tekgul H, Gokben S, Pehlivan S, Schalling M, et al. Multidrug resistance 1 (MDR1) gene polymorphisms in childhood drug-resistant epilepsy. J Child Neurol. 2010;25:1485–90. 31 210. Bournissen FG, Moretti ME, Juurlink DN, Koren G, Walker M, Finkelstein Y. Polymorphism of the MDR1/ABCB1 C3435T drug-transporter and resistance to anticonvulsant drugs: a meta-analysis. Epilepsia. 2009;50:898–903. 211. Dong L, Luo R, Tong Y, Cai X, Mao M, Yu D. Lack of association between ABCB1 gene polymorphisms and pharmacoresistant epilepsy: an analysis in a western Chinese pediatric population. Brain Res. 2011;1391:114–24. 212. Emich-Widera E, Likus W, Kazek B, Niemiec P, Balcerzyk A, Sieroń AL, et al. CYP3A5*3 and C3435T MDR1 polymorphisms in prognostication of drug-resistant epilepsy in children and adolescents. Biomed Res Int. 2013;2013:526837. 213. Haerian BS, Roslan H, Raymond AA, Tan CT, Lim KS, Zulkifli SZ, et al. ABCB1 C3435T polymorphism and the risk of resistance to antiepileptic drugs in epilepsy: a systematic review and meta-analysis. Seizure. 2010;19:339–46. 214. Haerian BS, Lim KS, Tan CT, Raymond AA, Mohamed Z. Association of ABCB1 gene polymorphisms and their haplotypes with response to antiepileptic drugs: a systematic review and meta-analysis. Pharmacogenomics. 2011;12:713–25. 215. Hung C-C, Tai JJ, Lin C-J, Lee M-J, Liou H-H. Complex haplotypic effects of the ABCB1 gene on epilepsy treatment response. Pharmacogenomics. 2005;6:411–7. 216. Kwan P, Wong V, Ng PW, Lui CHT, Sin NC, Poon WS, et al. Gene-wide tagging study of association between ABCB1 polymorphisms and multidrug resistance in epilepsy in Han Chinese. Pharmacogenomics. 2009;10:723–32. 217. Leschziner G, Jorgensen AL, Andrew T, Pirmohamed M, Williamson PR, Marson AG, et al. Clinical factors and ABCB1 polymorphisms in prediction of antiepileptic drug response: a prospective cohort study. Lancet Neurol. 2006;5:668–76. 218. Sánchez MB, Herranz JL, Leno C, Arteaga R, Oterino A, Valdizán EM, et al. Genetic factors associated with drug-resistance of epilepsy: relevance of stratification by patient age and aetiology of epilepsy. Seizure. 2010;19:93–101. 219. Siddiqui A, Kerb R, Weale ME, Brinkmann U, Smith A, Goldstein DB, et al. Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1. N Engl J Med. 2003;348:1442– 8. 220. Tan NCK, Heron SE, Scheffer IE, Pelekanos JT, McMahon JM, Vears DF, et al. Failure to confirm association of a polymorphism in ABCB1 with multidrug-resistant epilepsy. Neurology. 2004;63:1090–2. 221. Manna I, Gambardella A, Labate A, Mumoli L, Ferlazzo E, Pucci F, et al. Polymorphism of the multidrug resistance 1 gene MDR1/ABCB1 C3435T and response to antiepileptic drug treatment in temporal lobe epilepsy. Seizure. 2015;24:124–6. 222. Park WB, Choe PG, Song K-H, Jeon JH, Park SW, Kim HB, et al. Genetic factors influencing severe atazanavir-associated hyperbilirubinemia in a population with low UDP-glucuronosyltransferase 1A1*28 allele frequency. Clin Infect Dis. 2010;51:101–6. 223. Aarnoudse ALHJ, van Schaik RHN, Dieleman J, Molokhia M, van Riemsdijk MM, Ligthelm RJ, et al. MDR1 gene polymorphisms are associated with neuropsychiatric adverse effects of mefloquine. Clin Pharmacol Ther. 2006;80:367–74. 224. Ciccacci C, Borgiani P, Ceffa S, Sirianni E, Marazzi MC, Altan AMD, et al. Nevirapine-induced hepatotoxicity and pharmacogenetics: a retrospective study in a population from Mozambique. Pharmacogenomics. 2010;11:23–31. 225. Haas DW, Bartlett JA, Andersen JW, Sanne I, Wilkinson GR, Hinkle J, et al. Pharmacogenetics of nevirapine-associated hepatotoxicity: an Adult AIDS Clinical Trials Group collaboration. Clin Infect Dis. 2006;43:783–6. 32 226. Bozina N, Kuzman MR, Medved V, Jovanovic N, Sertic J, Hotujac L. Associations between MDR1 gene polymorphisms and schizophrenia and therapeutic response to olanzapine in female schizophrenic patients. J Psychiatr Res. 2008;42:89–97. 227. Lin Y-C, Ellingrod VL, Bishop JR, Miller DD. The relationship between P-glycoprotein (PGP) polymorphisms and response to olanzapine treatment in schizophrenia. Ther Drug Monit. 2006;28:668–72. 228. Kuzman MR, Medved V, Bozina N, Hotujac L, Sain I, Bilusic H. The influence of 5-HT(2C) and MDR1 genetic polymorphisms on antipsychotic-induced weight gain in female schizophrenic patients. Psychiatry Res. 2008;160:308–15. 229. Xing Q, Gao R, Li H, Feng G, Xu M, Duan S, et al. Polymorphisms of the ABCB1 gene are associated with the therapeutic response to risperidone in Chinese schizophrenia patients. Pharmacogenomics. 2006;7:987–93. 230. Mendoza JL, Urcelay E, Lana R, Martín MC, López N, Guijarro LG, et al. MDR1 polymorphisms and response to azathioprine therapy in patients with Crohn’s disease. Inflamm Bowel Dis. 2007;13:585–90. 231. Rustemoglu A, Gül Ü, Gümüş-Akay G, Gönül M, Yiğit S, Bozkurt N, et al. MDR1 gene polymorphisms may be associated with Behçet’s disease and its colchicum treatment response. Gene. 2012;505:333–9. 232. Saricaoglu H, Yilmaz M, Karkucak M, Ozturk HZY, Yakut T, Gulten T, et al. Investigation of ABCB1 gene polymorphism with colchicine response in Behçet’s disease. Genet Mol Res. 2011;10:1–6. 233. Tufan A, Babaoglu MO, Akdogan A, Yasar U, Calguneri M, Kalyoncu U, et al. Association of drug transporter gene ABCB1 (MDR1) 3435C to T polymorphism with colchicine response in familial Mediterranean fever. J Rheumatol. 2007;34:1540–4. 234. Grinyó J, Vanrenterghem Y, Nashan B, Vincenti F, Ekberg H, Lindpaintner K, et al. Association of four DNA polymorphisms with acute rejection after kidney transplantation. Transpl Int. 2008;21:879–91. 235. Hauser IA, Schaeffeler E, Gauer S, Scheuermann EH, Wegner B, Gossmann J, et al. ABCB1 genotype of the donor but not of the recipient is a major risk factor for cyclosporine-related nephrotoxicity after renal transplantation. J Am Soc Nephrol. 2005;16:1501–11. 236. Von Ahsen N, Richter M, Grupp C, Ringe B, Oellerich M, Armstrong VW. No influence of the MDR-1 C3435T polymorphism or a CYP3A4 promoter polymorphism (CYP3A4-V allele) on dose-adjusted cyclosporin A trough concentrations or rejection incidence in stable renal transplant recipients. Clin Chem. 2001;47:1048– 52. 237. Wang Y-Y, Zhang M, Lu F-M, Jiao Z, Qiu X-Y. CYP3A4 genetic polymorphisms predict cyclosporinerelated clinical events in Chinese renal transplant recipients. Chin Med J. 2012;125:4233–8. 238. Woillard J-B, Rerolle J-P, Picard N, Rousseau A, Guillaudeau A, Munteanu E, et al. Donor P-gp polymorphisms strongly influence renal function and graft loss in a cohort of renal transplant recipients on cyclosporine therapy in a long-term follow-up. Clin Pharmacol Ther. 2010;88:95–100. 239. Hebert MF, Dowling AL, Gierwatowski C, Lin YS, Edwards KL, Davis CL, et al. Association between ABCB1 (multidrug resistance transporter) genotype and post-liver transplantation renal dysfunction in patients receiving calcineurin inhibitors. Pharmacogenetics. 2003;13:661–74. 240. Klauke B, Wirth A, Zittermann A, Bohms B, Tenderich G, Körfer R, et al. No association between single nucleotide polymorphisms and the development of nephrotoxicity after orthotopic heart transplantation. J Heart Lung Transplant. 2008;27:741–5. 241. Yamauchi A, Ieiri I, Kataoka Y, Tanabe M, Nishizaki T, Oishi R, et al. Neurotoxicity induced by tacrolimus after liver transplantation: relation to genetic polymorphisms of the ABCB1 (MDR1) gene. Transplantation. 2002;74:571–2. 242. Yanagimachi M, Naruto T, Tanoshima R, Kato H, Yokosuka T, Kajiwara R, et al. Influence of CYP3A5 and ABCB1 gene polymorphisms on calcineurin inhibitor-related neurotoxicity after hematopoietic stem cell transplantation. Clin Transplant. 2010;24:855–61. 33 243. Zheng HX, Zeevi A, McCurry K, Schuetz E, Webber S, Ristich J, et al. The impact of pharmacogenomic factors on acute persistent rejection in adult lung transplant patients. Transpl Immunol. 2005;14:37–42. 244. Takatori R, Takahashi KA, Tokunaga D, Hojo T, Fujioka M, Asano T, et al. ABCB1 C3435T polymorphism influences methotrexate sensitivity in rheumatoid arthritis patients. Clin Exp Rheumatol. 2006;24:546–54. 245. Samara SA, Irshaid YM, Mustafa KN. Association of MDR1 C3435T and RFC1 G80A polymorphisms with methotrexate toxicity and response in Jordanian rheumatoid arthritis patients. Int J Clin Pharmacol Ther. 2014;52:746–55. 246. Drozdzik M, Rudas T, Pawlik A, Kurzawski M, Czerny B, Gornik W, et al. The effect of 3435C>T MDR1 gene polymorphism on rheumatoid arthritis treatment with disease-modifying antirheumatic drugs. Eur J Clin Pharmacol. 2006;62:933–7. 247. Chiou Y-H, Wang L-Y, Wang T-H, Huang S. Genetic polymorphisms influence the steroid treatment of children with idiopathic nephrotic syndrome. Pediatr Nephrol. 2012;27:1511–7. 248. Akin M, Turgut S, Ayada C, Polat Y, Balci YI, Erdoğan F. Relation between 3435C>T multidrug resistance 1 gene polymorphism with high dose methylprednisolone treatment of childhood acute idiopathic thrombocytopenic purpura. Gene. 2011;487:80–3. 249. Carlquist JF, Knight S, Horne BD, Huntinghouse JA, Rollo JS, Muhlestein JB, et al. Cardiovascular risk among patients on clopidogrel anti-platelet therapy after placement of drug-eluting stents is modified by genetic variants in both the CYP2C19 and ABCB1 genes. Thromb Haemost. 2013;109:744–54. 250. Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Méneveau N, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–75. 251. Singh M, Shah T, Adigopula S, Molnar J, Ahmed A, Khosla S, et al. CYP2C19*2/ABCB1-C3435T polymorphism and risk of cardiovascular events in coronary artery disease patients on clopidogrel: is clinical testing helpful? Indian Heart J. 2012;64:341–52. 252. Su J, Xu J, Li X, Zhang H, Hu J, Fang R, et al. ABCB1 C3435T polymorphism and response to clopidogrel treatment in coronary artery disease (CAD) patients: a meta-analysis. PLoS ONE. 2012;7:e46366. 253. Verschuren JJW, Boden H, Wessels JAM, van der Hoeven BL, Trompet S, Heijmans BT, et al. Value of platelet pharmacogenetics in common clinical practice of patients with ST-segment elevation myocardial infarction. Int J Cardiol. 2013;167:2882–8. 254. Xie C, Ding X, Gao J, Wang H, Hang Y, Zhang H, et al. The effects of CES1A2 A(-816)C and CYP2C19 loss-of-function polymorphisms on clopidogrel response variability among Chinese patients with coronary heart disease. Pharmacogenet Genomics. 2014;24:204–10. 255. Mega JL, Close SL, Wiviott SD, Shen L, Walker JR, Simon T, et al. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet. 2010;376:1312–9. 256. Wallentin L, James S, Storey RF, Armstrong M, Barratt BJ, Horrow J, et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320–8. 257. Hoenig MR, Walker PJ, Gurnsey C, Beadle K, Johnson L. The C3435T polymorphism in ABCB1 influences atorvastatin efficacy and muscle symptoms in a high-risk vascular cohort. J Clin Lipidol. 2011;5:91– 6. 258. Shabana MF, Mishriki AA, Issac MSM, Bakhoum SWG. Do MDR1 and SLCO1B1 polymorphisms influence the therapeutic response to atorvastatin? A study on a cohort of Egyptian patients with hypercholesterolemia. Mol Diagn Ther. 2013;17:299–309. 34 259. Becker ML, Visser LE, van Schaik RHN, Hofman A, Uitterlinden AG, Stricker BHC. Common genetic variation in the ABCB1 gene is associated with the cholesterol-lowering effect of simvastatin in males. Pharmacogenomics. 2009;10:1743–51. 35