Supplementary Material
advertisement

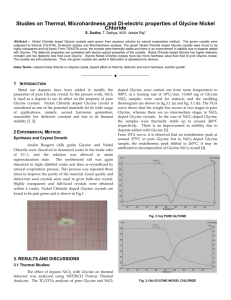
Supplementary Material for Quantitative Multi-Site Frequency Maps for Amide I Vibrational Spectroscopy Mike Reppert and Andrei Tokmakoff Glycine Charge Correction The appearance of the glycine charge discrepancy in the dipeptide data set, along with its correction is illustrated in Figure S1. The x-coordinate for each data point corresponds to the calculated electric field projected along the C=O bond axis and evaluated at the position of the O atom for an individual dipeptide MD simulation; the y-coordinate corresponds to the experimentally measured peak absorption frequency.1 The cluster of points near y = 1625 - 1640 cm-1 corresponds to measurements under basic conditions (deprotonated N-terminus), while the cluster between 1660 cm-1 and 1680 cm-1 corresponds to measurements under neutral and acidic conditions (protonated N-terminus). Green x’s indicate the absorption of amino acids of the form NH2-Gly-X-COO‒ under basic conditions; note their appearance significantly off-set from the best fit line for the remaining dipeptides. Red x’s indicate the same data points after introducing the charge correction described in the text during spectral calculations. Note that this correction appears to be necessary only under conditions in which the Gly amine group is charge neutral, i.e. not for protonated N-terminal groups. For clarity, the original and corrected atomic partial charges are listed in Table S1 below. The MD parameters for these simulations are similar to those reported in our earlier work1 (CHARMM27 force field with SPC/E water), but with a somewhat more accurate treatment of long-range electrostatics (Particle Mesh Ewald, with sodium and/or chloride ions added as necessary to balance charge). In addition, although for consistency with our previous work, the SPC/E water model was used for MD simulations, atomic partial charges were taken from the TIP3P model (more commonly used with the CHARMM27 force field) for calculating spectroscopic calculations (a charge of -0.834 eo on the water O atom and 0.417 eo on each water H atom). It should be emphasized that these charge corrections are applied only during post-processing, not during the MD run itself. Figure S1. Calculated electric field vs. experimental peak absorption frequency for dipeptides in our standard data set. Green x’s indicate dipeptides of the form NH2-Gly-Xxx-COO-. Red x’s indicate the same data points after the introduction of our Gly charge correction. Mid-chain NH2-Gly CA HA1 HA2 -0.02 (0.07) 0.10 (0.19) 0.09 (0.045) 0.09 (0.045) 0.09 (0.045) 0.09 (0.045) Table S1. Atomic partial charge assignments for the atoms CA, HA1, and HA2 in mid-chain and NH2-protonated N-terminal glycine residues. Each primary entry indicates the charge assigned by the CHARMM27 force field; numbers in parentheses indicate our corrected glycine charges used for spectroscopic calculations. All charges are in units of elementary charge eo. Charge assignments for other protonation states of the Gly residue (NH3+ N-terminal, and COO-/COOH C-terminal) are unmodified from the force field assignments. Linear relations for frequency shift coefficients We first provide a more precise expression for Eq. 3 in the main text: 𝐶𝐻 = −3.0640 𝐶𝑁 + 174.661. The best-fit equations for the correlation between CC, CO, and ωo parameters and the CN and CH coefficients are as follows: 𝐶𝐶 = −0.526374 𝐶𝑁 − 0.077332 𝐶𝐻 + 1089.106 𝐶𝑂 = −0.473626 𝐶𝑁 − 0.922668 𝐶𝐻 − 1089.106 𝜔𝑜 = 0.0317433 𝐶𝑁 + 0.0833667 𝐶𝐻 + 1701.11. In these expressions, all frequency shift coefficients are in units of cm-1. 𝑐𝑚−1 𝑒𝑜 , 𝐸𝐻 and all frequencies in References 1 M. Reppert and A. Tokmakoff, J. Chem. Phys. 138, 134116 (2013).