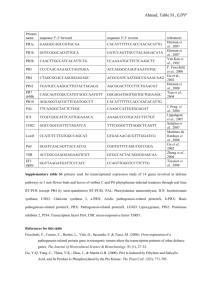
Causal agents associated with bacterial spot diseases of
advertisement

Biology and management of bacterial spot in tomato and pepper J. B. Jones, University of Florida, Plant Pathology Department, P.O. Box 110680, Gainesville, FL 32611 USA Abstract Bacterial spot disease on tomato and pepper is a significant problem in tropical, subtropical, and temperate climates. For many years this organism was identified as Xanthomonas campestris pv. vesicatoria. Genetic and phenotypic variation within this group of organisms associated with the diseases on these two plant species is well documented. Based on phenotypic and genotypic characterization, four distinct species have been identified. Two of the species, X. vesicatoria and X. euvesicatoria, are well documented as being present throughout the world. As a result of these two groups being the most prevalent of the four groups, they were logically the most studied in terms of taxonomic relationships and as such in 1995 were transferred from Xanthomonas campestris pv. vesicatoria to Xanthomonas axonopodis pv. vesicatoria and X. vesicatoria, respectively. X. axonopodis pv. vesicatoria was more recently transferred to X. euvesicatoria.The third species, X. perforans, which was originally isolated in 1991 in the United States and in Mexico, has more recently been found in many tomato growing regions in the United States and in the world including in Tanzania, South Korea and in the Indian Ocean region. The fourth species, X. gardneri, was originally isolated in Yugoslavia in 1957, and has since been found in Costa Rica, Brazil, the United States, Canada, in Tanzania and in the Indian Ocean region. Considerable work has been done on developing biological and chemical control strategies for managing bacterial spot on tomato and pepper. These strategies include using host resistance, plant activators, plant growth-promoting rhizobacteria, bacterial antagonists and bacteriophages. Host resistance has been a major focus of several research programs. The introgression of resistance genes into commercial tomato and pepper cultivars is a challenge because of the extreme pathogen variation and development of mutant strains. Considerable progress has been made in pepper to identify recessive resistance genes that appear to be more durable and less likely for the bacterium to overcome with the exception of X. gardneri strains which are aggressive on this resistance. In recent years biological control has been a focus of many research programs. Plant growth promoting rhizobacteria (PGPR) strains and bacterial antagonists have provided significant control of bacterial spot although results have not been consistent. Bacteriophages (viruses which kill target bacteria) have recently been used quite extensively in Florida for controlling bacterial spot on tomato. Phage therapy resulted in reduced disease and increased yield responses. Chemical control has relied for many years on copper compounds applied in combination with mancozeb to control copper tolerant bacterial strains. In recent years chemicals that induce systemic acquired resistance (SAR) have been developed. One of these compounds, Actigard, a widely used plant activator, has been effective in controlling bacterial spot on tomato. An integrated approach for controlling bacterial spot of tomato and pepper is recommended in which several strategies are combined to maximize the likelihood for disease control. 1 Causal agents associated with bacterial spot diseases of tomato and pepper Doidge (1921) in South Africa identified the pathogen causing bacterial spot disease on tomato (Solanum lycopersicum L. (Lycopersicum exculentum Mill.)) and designated it Bacterium vesicatorium. Gardner and Kendrick (1921) in the United States, also identified a bacterium causing a leaf spot of tomato and proposed naming it Bacterium exitiosum. Gardner and Kendrick noted that the only apparent difference between the strains was that the strains identified in the United States were strongly amylolytic while the strains isolated by Doidge were feebly amylolytic. Because of this minor difference between the two organisms, they deferred to B. vesicatorium because of a high degree of similarity between the two organisms and Doidge’s prior publication. In the 1990s, Vauterin et al. (1990 and 1995) and Stall et al. (1994) determined that two genetically and phenotypically distinct groups existed within X. campestris pv. vesicatoria. These groups were differentiated based on amylolytic and pectolytic activity along with pulsed-field gel electrophoresis (Stall et al., 1994). Bouzar et al. (1994a) compared a worldwide collection of 150 strains isolated from tomato and pepper and determined that the A strains were uniformly negative for starch hydrolysis and pectolytic activity while the B strains consisted of strongly amylolytic and pectolytic strains (Table 1). The groups were also separated based on protein profiles (Bouzar et al., 1994a). Furthermore, the A group consisted of only tomato race 1 (T1) strains and the B group of T2 strains (Bouzar et al., 1994b). Bouzar et al. (1994b) were also able to distinguish the strains serologically using a panel of monoclonal antibodies. Based on DNA-DNA hybridization tests (Vauterin et al., 1995; Stall et al., 1994), it was evident that group A and B strains were not related at the species level. Vauterin et al. (1995) proposed a reclassification of the xanthomonads and divided X. campestris pv. vesicatoria into two species, with the B strains being retained in X. vesicatoria and the A strains placed as a pathovar of X. axonopodis. Later two other genetically distinct xanthomonads were identified that were associated with tomato. The first was isolated in 1957 by Sutic (1957) who identified a bacterium on tomato in Yugoslavia and named it Pseudomonas gardneri. We have since identified the same organism in Costa Rica in the early 1990s based on REP-PCR (Bouzar et al., 1999) and more recently determined the strains to be members of the same species (Jones et al., 2000). This organism has since been identified in Brazil (Quezado-Duval et al., 2004) and has also been reported in the United States (Cuppels et al., 2006). The second unique group consisted of T3 strains that were isolated in Florida in the United States in the early 1990s (Jones et al., 1995). Besides their response on the tomato differentials, these strains were different from the other strains based on serology and pulsed-field gel electrophoresis (Bouzar et al., 1996; Jones et al., 2000). Jones et al. (2000) characterized these two types of strains and determined that they were new groups, C and D based on characteristics listed in Table 1. Group C represented by T3 strains originally were determined to be most closely related to the A group based on DNA-DNA hybridization experiments and were thus placed within X. axonopodis pv. vesicatoria, whereas the D 2 group represented by strains including the one originally identified by Sutic (1957) were genetically distinct from the other three groups of xanthomonads associated with tomato. We presented data showing that four distinct Xanthomonas species cause bacterial diseases on pepper and tomato (Jones et al., 2004). We elevated X. axonopodis pv. vesicatoria to species status naming it X. euvesicatoria in order to avoid confusion with what is now classified as X. vesicatoria (i.e., the B group of strains). Furthermore, we provided evidence for naming two new species that represent the C and D groups of xanthomonads associated with tomato. The C group of strains have transferred to X. perforans (Jones et al., 2004). The specific epithet was selected as it describes the symptoms associated with disease of tomato leaves caused by this bacterium in which oftentimes holes develop in the leaf following lesion development (Figure 1D). The D group strains elevated to species status and placed in X. gardneri (Jones et al., 2004) using the specific epithet originally proposed by Sutic (1957). We have also seen changes in bacterial populations in Florida relating to changes in host genotype and also as a result of a competitive advantage of certain strains over other strains. On pepper, seed companies have released cultivars were contained the Bs2. Prior to deployment of this resistance, all bacterial strains in Florida associated with bacterial spot of pepper were avirulent on genotypes containing Bs2 (Bouzar et al., 1994b). Within years of deployment of this resistance strains were isolated that were aggressive on this resistance (Gassmann et al., 2000). Currently, only strains which do not interact with Bs2 are isolated from pepper cultivars in Florida where most of the current cultivars continue to contain Bs2. On tomato in Florida, prior to 1991, only A group strains (X. euvesicatoria) were present. Fields were surveyed throughout Florida and a low incidence of group C strains (X. perforans) were identified (Jones et al., 1995). We observed in certain fields that C strains became prevalent and displaced A strains (Jones et al., 1998). As a result of characterization of C strains we identified several compounds termed bacteriocins that are toxic to A strains but nontoxic to C strains (Tudor-Nelson et al., 2003). Our laboratory determined that the bacteriocins were in part responsible for the competitive advantage of C strains over A strains (Hert et al., 2005). As of 2006, we surveyed 20 tomato fields in Florida and collected 377 strains which were all identified as X. perforans. On tomato, X. perforans has displaced X. euvesicatoria. Currently, we only have X. perforans on tomato (Horvath et al., 2012). Symptoms On tomato the bacterium infects the leaves, stems, and fruit, causing spots that are generally brown and circular (Fig.1A and D). The spots may appear watersoaked when observed during rainy weather or early in the morning when dew is present. Lesions often do not exceed 3 mm in diameter. Lesions may easily be confused with bacterial speck, early blight, gray leafspot, or target spot. Unlike target spot and early blight, bacterial spot lesions do not have concentric zones. Furthermore, the lesions are generally darker in 3 color and less uniformly distributed than gray leafspot lesions. Often, a prominent halo surrounding pinpoint lesions is present with target spot and early blight but not with bacterial spot lesions. When conditions are optimal for disease development, spots on the leaves, petioles, and the rachis will coalesce to form long dark streaks. A general yellowing may occur on leaflets with many lesions. Blighting of the foliage occurs with the lesions coalescing. The dead foliage remains on the plant, giving it a scorched appearance (Fig. 1C). Fruit lesions begin as minute, slightly raised blisters. As a spot increases in size it becomes brown in color, scab-like, and slightly raised (Fig. 1B). However, lesions may also be raised around the margins but sunken in the middle. A developing lesion may have a faint to prominent halo which eventually disappears. On pepper, the bacterium affects above ground plant parts, producing small, brown, watersoaked lesions that become brown with necrotic centers (Fig. 2). The spots are watersoaked when observed during rainy weather or in the early morning when dew is present. Lesions rarely develop to more than 3 mm in diameter. The lesions are generally sunken on the top surface and slightly raised on the bottom. When conditions are optimal for disease development, spots on the leaves will coalesce and large blighted areas appear. This is often followed by yellowing on leaves which if often followed by premature leaf drop (Figure 2). Fruit lesions begin as circular green spots. As a spot increases in size it becomes brown in color, and has a cracked, roughened wart-like appearance similar to the fruit spots on tomato and can range in size from 2 to 3 mm in diameter. Integrated management of bacterial spot Tomato bacterial spot, caused by Xanthomonas campestris pv. vesicatoria (Doidge) Dye (Xcv), is a major threat to tomato (Lycopersicon esculentum Mill.) commercial production (Jones et al., 1991). Chemical control of bacterial spot has relied over the years on multiple applications of copper- or streptomycin-based bactericides (Conover and Gerhold, 1981; Jones and Jones, 1985; Stall and Thayer, 1962). Although chemicals have been used extensively for controlling bacterial spot, there have been significant limitations associated with their use. Streptomycin was used extensively in the 1950s, but resistant bacterial strains developed and rendered it ineffective (Quezado-Duval et al., 2003; Stall and Thayer, 1962). A similar phenomenon was observed with copper (Stall et al., 1986). As a result of the build-up in copper-tolerant strains, a mixture of copper and ethylene-bisdithiocarbamates has been used to control these strains (Conover and Gerhold, 1981; Marco and Stall, 1983). Although copper-mancozeb combinations reduced bacterial leaf spot on tomato leaf surfaces (Jones et al., 1991) and resulted in improved disease control, the combination was ineffective when weather conditions were optimal for disease development and positive yield responses are rarely observed in situations where coppertolerant strains were present (Jones and Jones, 1985). Although a number of control practices are available (Jones et al., 1991), these strategies have not proven highly effective when weather conditions favor disease development. Therefore, alternative disease control strategies are needed that result in improved disease control and yield responses. Alternative strategies that have been tested 4 and which were associated with a reduction in disease severity of bacterial spot and bacterial speck of tomato include introgression of resistant genes into commercial varieties, activation of natural plant defense mechanisms by systemic acquired resistance (SAR) inducers (Louws et al., 2001; Romero et al., 2001) and PGPRs (Ji et al., 20066), application of antagonistic bacteria (Wilson et al., 2002) and bacteriophages (Balogh et al., 2003; Flaherty et al., 2000). Host resistance to pathogens is a critical component of integrated disease management systems. Plant resistance has been the focus of many research programs for controlling bacterial spot of tomato (Scott et al., 1995, 1997, 2003; Scott and Jones, 1986 and 1989). Although resistance genes have been identified and introgressed into tomato and pepper genotypes with good horticultural qualities, shifts in races of Xcv offset breeders’ efforts to develop commercial tomato cultivars (Jones et al., 1998; Kousik and Ritchie, 1996). Tomato races T1 and T2 were first identified by Wang et al. (1990) and were determined to be worldwide in distribution (Bouzar et al., 1994b). A limited collection from Europe and Africa revealed only T1 strains, whereas intensive sampling revealed only T1 strains present in six Caribbean islands and T1 and T2 strains present in Central America (Bouzar et al., 1999 ). Florida contained only T1 strains isolated prior to 1991 (Stall, unpublished). T2 has been isolated in several other states within the United States including California, Oklahoma, Indiana, Ohio, and Georgia (Bouzar et al., 1994b). More recently T3 was identified in Florida (Jones et al., 1995) and has since been identified in Mexico (Bouzar et al., 1996), other states in the U.S.A. and other countries including Brazil (QuezadoDuval and Camargo, 2004). Recently, we have isolated a new race, designated T4 (Minsavage et al., 2003), that is a mutated T3 strain, which no longer interacts with the Xv3 gene, but that does interact with the Xv4 gene in Lycopersicon pinnellii LA716 (Astua-Monge et al., 2000). In that same study, Minsavage et al. (2003) identified T2 strains which were determined to be C group strains based on sequence analysis of the hrp region (Obradovic et. al., 2004). These strains contained mutations in avrXv3 and avrXv4 (Minsavage et al., 2003). We are continuing to look for resistance that is not associated with a hypersensitive reaction such as identified in PI114490 by Scott et al. (2003). We are attempting to identify other sources and combine with this resistance such that bacterial spot is significantly limited. Quantitative and qualititative resistance has been identified in pepper to the bacterial spot pathogen (Adamson and Sowell, 1983; Cook and Guevara, 1984; Cook and Stall, 1963; Hibberd et al., 1987; Hibberd, 1989; Kim and Hartman, 1985). Qualitative resistance has been deployed extensively in commercial cultivars. Three dominant genes for qualitative resistance to bacterial spot in peppers have been identified (Cook and Guevara, 1984; Cook and Stall, 1963; Kim and Hartman, 1985) and are referred to as Bs1, Bs2, and Bs3., which interact in a gene-for-gene manner with the corresponding avirulence gene (Minsavage et al., 1990). In Florida many of the commercial pepper cultivars contain the Bs2 gene which interacts with the corresponding avrBs2 in the bacterium. Previousy it had been shown that avrBs2 was a virulence factor and thus it was hypothesized that resistance associated with Bs2 would be durable given that the avirulence gene was necessary for increased virulence (Kearney and Staskawicz, 1990). 5 Upon introduction of commercial varieties with the Bs2 races of the pathogen began appearing in which the avrBs2 did not interact with the Bs2 in an incompatible manner (Ritchie et al., 1998; Kousik and Ritchie, 1999). Gassmann et al. (2000) determined that many of the mutations in the avrBs2 were point mutations resulting in single amino acid changes and that several of these did not affect aggressiveness of the strain. Therefore the durability of the resistance appears to have been compromised. More recently a source of resistance was identified in pepper that was associated with Capsicum baccatum. The resistance was determined to be a dominant gene and was designated Bs7 and was determined to interact with avrBs1.1 and avrBs7 in X. euvesicatoria and X. gardneri, respectively (Potnis et al., 2012). As a result of the breakdown in resistance to these dominant genes in pepper, considerable effort has been made in identifying other types of resistance. Two recessive genes, bs5 and bs6, have been identified which confer a significant level of resistance to all known races of the pathogen including pepper race 6 (Jones et al., 2002). Attempts to mutagenize the strains to become virulent on these two genes have been unsuccessful. These results are encouraging and may indicate that this resistance is durable. Although this may be durable resistance, it would be prudent that this resistance be used in combination with the three dominant genes. Recently, we determined that X. gardneri (D group) strains are virulent on these two recessive resistance genes (Jones et al., unpublished results); given that these X. gardneri strains contain avirulence genes which interact with Bs2 and Bs3, cultivars containing the two recessive resistance genes that are deployed in regions where X. gardneri is present should also contain the two dominant resistance genes (I.e, Bs2, Bs3). Foliar bacterial biological control agents and plant growth promoting rhizobacteria (PGPR) have been tested for control of bacterial spot of tomato (Wilson et al., 2006). In field trials foliar biological control agents and PGPR strains controlled bacterial spot although they provided variable results. PGPR strains may induce plant resistance under field conditions, providing effective suppression of bacterial spot of tomato. As a result of that study, there may be some benefit for integrating rhizosphere-applied PGPR and foliarapplied biological control agents into a bacterial spot management program. Plant activators have been shown to induce systemic acquired resistance (SAR) in plants to limit pathogenesis of many plant pathogens, including fungi, viruses and bacteria. Acquired resistance in plants has been observed for many years and can be induced by necrotizing or incompatible pathogens, biological elicitors, and certain chemicals (Godard et al., 1999; Hammerschmidt and Kuc, 1995; Sticher et al., 1997). One widely used compound, acibenzolar-S-methyl (CGA 245704, Actigard 50WG, Bion 50WG, Syngenta, Basel, Switzerland), which by definition acts as a plant activator (Kessmann et al, 1994) has been used extensively on tomatoes for control of bacterial spot and bacterial speck (Louws et al. 2001). In field studies where Actigard was applied, bacterial spot disease severity was significantly reduced in fresh-market and processing tomatoes without significant yield responses. In another study conducted in Florida, Actigard effectively reduced disease without significantly improving yields (Obradovic et 6 al., 2005). In greenhouse studies applications of Actigard stimulated defense responses as manifested by development of necrotic lesions on leaves which in further tests were confirmed to result from a hypersensitive response (Obradovic et al., 2004). Given that Actigard applications activate strong defense responses, further research is needed on application rates and timing to reduce negative effects on yield responses. Romero and Ritchie (2004) observed that Actigard effectively delayed race changes and may increase the durability of genotype-specific resistance. Therefore, deployment of varieties with moderate to high levels of resistance to bacterial diseases coupled with the use of SAR inducers may increase durability of these resistances. SAR inducers in tomato show significant promise for future disease management. Practices that help reduce disease pressure, such as the use of resistant cultivars, play an important part in limiting the development of resistance in pathogen populations and should always be used in conjunction with an effective management program. Bacteriophages, have significant potential as biocontrol agents for controlling bacterial diseases pathogens (Jones, 2012). However, phages were abandoned due to the emergence of bacterial mutants resistant to the phages. An approach was used in which mixtures of bacteriophages were used to overcome problems experienced in the past (Jackson, 1989). Mixtures of bacteriophages were tested for control of the bacterial spot disease on tomato on overhead irrigated tomato transplants and observed that irrigation water containing bacteriophages specific to the bacterial spot pathogen reduced disease incidence compared to a copper bactericide (Somodi et al., 1997). Furthermore, in several field studies, bacterial spot severity was significantly reduced on phage-treated plants compared to those receiving copper-mancozeb or the control (Flaherty et al., 2000; Obradovic et al., 2004), while yield was also significantly improved. The efficacy of bacteriophages was improved by identifying compounds that could be added to phage suspensions and that significantly enhanced longevity of phages under greenhouse and field conditions resulting in greater efficacy against the bacterial spot pathogen (Balogh et al., 2003, Obradovic et al., 2004). Several formulations have been identified that enhance bacteriophage longevity on leaf surfaces in the greenhouse (Balogh et al., 2003). The formulation which has preven quite effective contains powdered skim milk and sucrose. The combination when applied with bacteriophage extended the duration of phage activity on the leaf surface significantly compared to the nonformulated treatment. In field tests, all formulations except the non-formulated phage significantly reduced disease severity attributed to bacterial spot compared to the control (Balogh et al., 2003). All bacteriophage treatments were significantly better than the standard copper-mancozeb treatment. Furthermore, the timing of phage application affected efficacy (Balogh et al., 2003). Phage populations were maintained at significantly higher levels when applied as formulated phage as compared as non-formulated phage (Iriarte et al., 2006).Phage applications made in the mid-morning (10 AM) resulted in a drastic reduction in phage populations on the leaf surface within hours. Balogh et al. (2003) determined that phage applied in the evening provided significantly better control than phage applied in the morning. An additional factor that is essential when using bacteriophages is that the bacterial strains in the field be assayed early in the cropping season as to sensitivity to the phage. Bacterial strains differ considerably in terms of sensitivity to specific phages and it 7 is critical that the phages used in a particular field are virulent on the bacterial strains in that field. Effective disease control of bacterial spot of tomato and pepper will require an integrated approach. Control of bacterial spot with conventional copper bactericides has proven to be extremely difficult given the presence of resistant strains in the bacterial population. As a result of the development of modified management strategies (i.e., plant resistance, plant activators, biological control agents, bacteriophages), growers have more opportunities to control bacterial spot of pepper and tomato. Literature Cited Adamson, W. C., and Sowell, G. 1983. Inheritance of bacterial spot resistance in pepper. HortScience 18:905-906. Astua-Monge, G., G. V. Minsavage, R. E. Stall, C. E. Vallejos, M. J. Davis, and J. B. Jones. 2000. Xv4-avrXv4: A new gene-for-gene interaction identified between Xanthomonas campestris pv. vesicatoria race T3 and the wild tomato relative Lycopersicon pennellii Mol. Plant-Micro. Interact. 13:1346-1355. Balogh, B. , Jones, J. B., Momol, M. T., Olson, S. M., Obradovic, A. , Jackson, L. E. 2003. Improved Efficacy of Newly Formulated Bacteriophages for Management of Bacterial Spot on Tomato. Plant Dis. 87:949-954. Bouzar, H., Jones, J. B., Minsavage, G. V., Stall, R. E., & Scott, J. W. 1994a. Proteins unique to phenotypically distinct groups of Xanthomonas campestris pv. vesicatoria revealed by silver staining. Phytopathology 84:39-44 Bouzar, H., Jones, J. B., Somodi, G. C., Stall, R. E., Daouzli, N., Lambe, R. C., FelixGastelum, R., & Trinidad-Correa, R.. 1996. Diversity of Xanthomonas campestris pv. vesicatoria in tomato and pepper fields of Mexico. Can. J. Plant Pathol. 18:75-77. Bouzar, H., Jones, J. B., Stall, R. E., Hodge, N. C., Minsavage, G. V., Benedict, A. A., & Alvarez, A. M. 1994b. Physiological, chemical, serological, and pathogenic analyses of a worldwide collection of Xanthomonas campestris pv. vesicatoria strains. Phytopathology 84:663-671. Bouzar, H., J. B. Jones, R. E. Stall, F. J. Louws, M. Schneider, J.L.W. Rademaker, F. J. de Bruijn, and L. E. Jackson. 1999. Multiphasic analysis of xanthomonads causing bacterial spot disease on tomato and pepper in the Caribbean and Central America : evidence for common lineages within and between countries. Phytopathology 89:328-335. Conover, R.A., and Gerhold, N.R. 1981. Mixtures of copper and maneb or mancozeb for control of bacterial spot of tomato and their compatibility for control of fungus diseases Phytophthora infestans, Stemphylium solani, Xanthomonas campestris pv. vesicatoria, Florida . Proc. Fla. State Hort. Soc. 94:154-156 Cook, A. A., and Guevara, Y. G. 1984. Hypersensitivity in Capsicum chacoense to race 1 of the bacterial spot pathogen of pepper. Plant Dis. 68:329-330. Cook, A. A., and Stall, R. E. 1963. Inheritance of resistance in pepper to bacterial spot. Phytopathology 53:1060-1062 8 Cuppels, D. A., Louws, F. J., and Ainsworth, T. 2006. Development and evaluation of PCRbased diagnostic assays for the bacterial speck and bacterial spot pathogens of tomato. Plant Dis. 90:451-458. Doidge, E. M.: A tomato canker. 1921. Ann. Appl. Biol. 7:407-430. Dye, D. W.: Cultural and biochemical reaction of additional Xanthomonas species. 1966. New Zealand J. Sci. 9:913-919. Gardner, M. W, & Kendrick, J. B.: Bacterial spot of tomato. 1921. J. Agr. Res. 21:123156. Godard, J. F., Ziadi, S., Monot, C., Le Corre, D., and Silue, D. 1999. Benzothiadiazole (BTH) induces resistance in cauliflower (Brassica oleracea var. botrytis) to downy mildew of crucifers caused by Peronospora parasitica. Crop Prot. 18:397-405. Hammerschmidt, R., and Kuc, J., eds. 1995. Induced resistance to disease in plants. Kluwer Scientific Publishers, Dordrecht, Netherlands. Hert, A. P., P. D. Roberts, M. T. Momol, G. V. Minsavage, S. M. Tudor-Nelson, and J. B. Jones. 2005. Relative Importance of Bacteriocin-Like Genes in Antagonism of Xanthomonas perforans Tomato Race 3 to Xanthomonas euvesicatoria Tomato Race 1 Strains. Appl. Env. Microbiology. 71:3581-3588. Hibberd, A. M. 1989. Quantitative resistance to bacterial leaf spot of pepper compared in mono and polycyclic disease progress tests. Pages 213-219 in: Tomato and Pepper Production in the Tropics: Integrated Management Practices. Asian Vegetable Research and Development Center, Shanhua, Tainan. Hibberd, A. M., Stall, R. E., and Bassett, M. J. 1987. Different phenotypes associated with incompatible races and resistance genes in bacterial spot disease of pepper. Plant Dis. 71:1075-1078. Horvath DM, Stall RE, Jones JB, Pauly MH, Vallad GE, et al. (2012) Transgenic Resistance Confers Effective Field Level Control of Bacterial Spot Disease in Tomato. PLoS ONE 7(8): e42036. doi:10.1371/journal.pone.0042036. Iriarte, F., Balogh, B., Momol, T. M., Smith, L. M., Wilson, M., and Jones, J. B. 2007. Factors affecting survival of bacteriophage on tomato leaf surfaces. Appl. & Env. Microbiol. 73:1704-1711. Ji, P., H.L. Campbell, J.W. Kloepper, J.B. Jones, T.V. Suslow, M. Wilson. 2006. Integrated biological control of bacterial speck and spot of tomato under Weld conditions using foliar biological control agents and plant growth-promoting rhizobacteria. Biological Control 36:358-367. Jones, J. B., R. E. Stall, and H. Bouzar. 1998. Diversity among xanthomonads pathogenic on pepper and tomato. Annu. Rev. Phytopathol. 36:41-58. Annual Reviews Inc. Palo Alto. Jones, J. B., H. Bouzar, R. E. Stall, E.C. Almira, P. Roberts, B.W. Bowen, J. Sudberry, P. Strickler, & J. Chun.2000. Systematic analysis of xanthomonads (Xanthomonas spp.) associated with pepper and tomato lesions. Int. J. Syst. Bacteriol. 50:1211-1219. Jones, J. B., and J. P. Jones. 1985. The effect of bactericides, tank mixing time, and spray schedule on bacterial leaf spot of tomato. Proc. Fla. State Hort. Soc. 98:244-247. Jones, J. B., J. P. Jones, R. E. Stall, and T. A. Zitter (eds.). 1991. Compendium of Tomato Diseases. APS Press, St. Paul, MN. 9 Jones, J. B., Lacy, G. H., Bouzar, H., Stall, R. E., and Schaad, N. W. 2004. Reclassification of the xanthomonads associated with bacterial spot disease of tomato and pepper. Syst. & Appl. Micro. 27:755-762. Jones, J.B., Vallad, G.E., Iriarte, F.B., Obradović, A., Wernsing, M.H., Jackson, L.E., Balogh, B., Hong, J.C., and Momol, MT. 2012. Considerations for using bacteriophages for plant disease control. Bacteriophage. 2, 208-214. Jones, J. B., Minsavage, G. V., Roberts, P. D., Johnson, R. R., Kousik, C. S., Subramanya, S. , Stall, R. E. 2002. A Non-Hypersensitive Resistance in Pepper to the Bacterial Spot Pathogen is Associated with Two Recessive Genes. Phytopathology. 92:273 -277. Jones, J. B., R. E. Stall, G. C. Somodi, H. Bouzar, N. C. Hodge. 1995. A third tomato race of Xanthomonas campestris pv. vesicatoria. Plant Dis. 79:395-398. Jones, J. B., S. S. Woltz, J. P. Jones, and K. L. Portier. 1991. Population dynamics of Xanthomonas campestris pv. vesicatoria on tomato leaflets treated with copper bactericides. Phytopathology 81:714-719. Kearney, B., and B. J. Staskawicz. 1990. Widespread distribution and fitness contribution of Xanthomonas campestris avirulence gene avrBs2. Nature 346:385–386 Kessmann, H., Staub, T., Ligon, J., Oostendorp, M., and Ryals, J. 1994. Activation of systemic acquired disease resistance in plants. Eur. J. Plant Pathol. 100:359-369. Kim, B.-S., and Hartmann, R. W. 1985. Inheritance of a gene (Bs3) conferring hypersensitive resistance to Xanthomonas campestris pv. vesicatoria in pepper (Capsicum annuum). Plant Dis. 69:233-235. Kousik C. S. and D. F. Ritchie. 1999. Development of Bacterial Spot on Near-Isogenic Lines of Bell Pepper Carrying Gene Pyramids Composed of Defeated Major Resistance Genes. Phytopathology 89:1066-1072. Kousik, C. S., and Ritchie, D. F. 1999. Development of bacterial spot on near-isogenic lines of bell pepper carrying gene pyramids composed of defeated major resistance genes. Phytopathology 89:1066-1072. Kousik, C. S. and Ritchie, D. F. 1996. Race shift in Xanthomonas campestris pv. vesicatoria within a season in field-grown pepper. Phytopathology 86: 952-958. Louws, F. J., Wilson, M., Campbell, H. L., Cuppels, D. A., Jones, J. B., Shoemaker, P. B., Sahin, F., and Miller, S. A. 2001. Field control of bacterial spot and bacterial speck of tomato using a plant activator. Plant Dis. 85:481-488. Marco, G. M., and Stall, R. E. 1983. Control of bacterial spot of pepper initiated by strains of Xanthomonas campestris pv. vesicatoria that differ in sensitivity to copper. Plant Dis. 67:779- 781. Minsavage, G. V., B. Balogh, R. E. Stall, and J. B. Jones. 2003. New tomato races of Xanthomonas campestris pv. vesicatoria associated with mutagenesis of tomato race 3 strains. Phytopathology 93:S62. Minsavage, G. V., Dahlbeck, D., Whalen, M. C., Kearney, B., Bonas, U., Staskawicz, B. J., and Stall R. E. 1990. Gene-for-gene relationships specifying disease resistance in Xanthomonas campestris pv. vesicatoria -pepper interactions. Mol. Plant-Microbe Interact. 3:41-47. 10 Obradovic, A., Jones, J. B., Momol, M. T., Balogh, B., Olson, S. M. 2004. Management of Tomato Bacterial Spot in the Field by Foliar Applications of Bacteriophages and SAR Inducers. Plant Disease, 88: 736-740. Obradovic, A., Jones, J. B., Momol, M. T., Olson, S. M., Jackson, L. E., Balogh, B., Guven, K., and Iriarte, F. B. 2005. Integration of biological control agents and systemic acquired resistance inducers against bacterial spot on tomato. Plant Dis. 89:712-716. Obradovic, A. O., Mavridis, Rudolph, K., Janse, J., Arsenijevic, Jones, J. B., Minsavage, G. V., and Wang, J.F. 2004 Characterization and PCR-based typing of Xanthomonas campestris pv. vesicatoria pepper and tomato pathogen in Serbia. Eur. J. Plant Path. Potnis, Neha, Gerald Minsavage, J. Kennon Smith, Jason C. Hurlbert, David Norman, Rosana Rodrigues, Robert E. Stall, and Jeffrey B. Jones. Avirulence proteins AvrBs7 from Xanthomonas gardneri and AvrBs1.1 from Xanthomonas euvesicatoria contribute to a novel gene-for-gene Interaction in Pepper. Mol. Plant-Microbe Interact. 25:307-320. Quezado-Duval, A. . M., and Camargo, L. E. A. 2004. Races of Xanthomonas spp. associated to bacterial spot in processing tomatoes in Brazil. Hortic. Bras.22:80-86. Quezado-Duval, A. M., Filho, A. G., Leite, R. P., Jr., and L. E. A. Camargo. 2003. Sensibbilidade a cobre, estreptomicina e oxitetraciclina em Xanthomonas spp. associadas à mancha-bacteriana do tomate para processamento industrial. Hortic. Bras. 21:670-675. Quezado-Duval, A. M., Leite, R. P., Jr., Truffi, D., and L. E. A. Camargo. 2004. Outbreaks of bacterial spot caused by Xanthomonas gardneri on processing tomato in central-west Brazil. Plant Dis. 88:157-161. Ritchie, D. F., Kousik, C. S., and Paxton, T. C. 1998. Response of bacterial spot pathogen strains to four major resistance genes in pepper. Page 14 in: Proc. Natl. Pepper Conf. B. Villalon and L. Brandenberger eds. Citrus and Vegetable Magazine, Tampa, FL. Romero, A.M., Kousik, C.S., Ritchie, D.F., 2001. Resistance to bacterial spot in bell pepper induced by acibenzolar-S-methyl. Plant Dis. 85, 189–194. Scott, J. W., D. M. Francis,, S. A. Miller, G. C. Somodi, and J. B. Jones. 2003. Tomato bacterial spot resistance derived from PI114490; inheritance of resistance to race T2 and relationship across three pathogen races. J. Amer. Hort. Sci. 128:698-703. Scott, J. W., and J. B. Jones. 1989. Inheritance of resistance to foliar bacterial spot of tomato incited by Xanthomonas campestris pv. vesicatoria. J. Amer. Soc. Hort. Sci. 114:111-114. Scott, J. W., and J. B. Jones. 1986. Sources of resistance to bacterial spot (Xanthomonas campestris pv. vesicatoria (Doidge)Dye) in tomato. Hortsci. 21:304-306. Scott, J. W., J. B. Jones, G. C. Somodi, and R. E. Stall. 1995. Screening tomato accessions for resistance to Xanthomonas campestris pv. vesicatoria, race T3. HortScience 30(3):579-581. Scott, J. W., Miller, S. A., Stall, R. E., J. B. Jones, G. C. Somodi, V. Barbosa, D. L. Francis, and F. Sahin. 1997. Resistance to race T2 of the bacterial spot pathogen in tomato. HortScience 32:724-727 Stall, R. E., Beaulieu, C., Egel, D., Hodge, N. C., Leite, RP, Minsavage, G. V., Bouzar, H., Jones, J. B., Alvarez, A. M., & Benedict, A. A.1994. Two genetically diverse groups of strains are included in Xanthomonas campestris pv. vesicatoria. Int. J. Syst. Bacteriol. 44:47-53. 11 Stall, R. E., D. C. Loschke, and J. B. Jones. 1986. Linkage of copper resistance and avirulence loci on a self-transmissible plasmid in Xanthomonas campestris pv. vesicatoria. Phytopathology 76:240-243. Stall, R. E., and Thayer, P. L. 1962. Streptomycin resistance of the bacterial spot pathogen and control with streptomycin. Plant Dis. Rep. 46:389-392. Sticher, L., Mauch-Mani, B., and M´etraux, J. P. 1997. Systemic acquired resistance. Annu. Rev. Phytopathol. 1997. 35:235–70 Šutic, D.: Bakterioze crvenog patlidzana (Tomato bacteriosis). 1957. Posebna Izd. Inst. Zasht. Bilja Beograd (Spec. Edit. Inst. Plant Prot. Beograd) 6:1-65. English summary: Rev. Appl. Mycol. 36:734-735. Tudor-Nelson, S. M, G.V. Minsavage, R.E. Stall and J.B. Jones. 2003. BacteriocinLike Substances from Tomato Race 3 Strains of Xanthomonas campestris pv. vesicatoria. Phytopathology 93: 1415-1421. Vauterin, L., J. Swings, L. Vauterin, J. Swings, K. Kersters, M. Gillis, T. W. Mew, M. N. Schroth, , N. J. Palleroni, D. C. Hildebrand, D. E. Stead, E. L. Civerolo, A. C. Hayward, H. Maraite, R. E. Stall, J. F. Bradbury. 1990. Towards an improved taxonomy of Xanthomonas. Int. J. Syst. Bacteriol. 40:312-316. Vauterin, L. Hoste, B., Kersters, K. and Swings, J.1995. Reclassification of Xanthomonas. Int. J. Syst. Bacteriol. 45:472-489. Wang, J-F, Jones, JB, Scott, J. W., Stall, RE. 1990. A new race of the tomato group of strains of Xanthomonas campestris pv. vesicatoria. (Abstr.) Phytopathology 80:1070. Wilson, M. , Campbell, H. L., Jones, J. B., Cuppels, D. L. 2002. Biological Control of Bacterial Speck of Tomato Under Field Conditions at Several Locations in North America. Phytopathology. 92:1284 -1292. 12 Table 1. Phenotypic groups of Xanthomonas species associated with tomato and pepper Phenotypic Tomato Race Distribution Mab1 Protein2 PFGE3 group Amylolytic Pectate activity4 hydrolysis4 X. euvesicatoria A T1 Worldwide 1, 21 32 kDa A -5 - X. vesicatoria B T2 Worldwide 8, 15 27 kDa B + + X. perforans C T3 Brazil, Mexico, 30 27 kDa C + + 8 27 kDa D - - Thailand, X. gardneri D T3 and T4 U.S.A. T1 Costa Rica, USA Yugoslavia, Brazil 1 Monoclonal antibody developed using X. c. vesicatoria strains that reacts in enzyme linked immunosorbent assay (4, 14). Protein patterns based on study by Bouzar et al. (2) 3 Pulse field gel electrophoresis group (Jones et al (2000)). 4 + = starch hydrolysis and pectate hydrolysis visible in less than 2 day (Bouzar et al. (1994a)). 5 Several strains from Mexico and Ohio, U.S.A. are positive for amylolytic activity (Bouzar et al. (1996) 2 13 A B C D Figure 1. A, B, and C, Bacterial spot of tomato on leaflets, fruit spot, and blighting in field, respectively, caused by Xanthomonas euvesicatoria; D, Bacterial spot on tomato leaflet caused by Xanthomonas perforans (Note shot-hole symptom) 14 A B Figure 3A. Close-up of bacterial spot naturally infected pepper leaves in the field. B. Field symptoms showing leaf spot, blighting and defoliation. 15