Brayton
advertisement

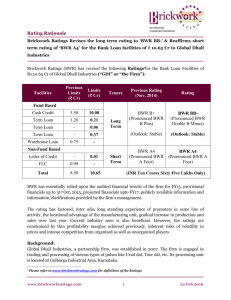
Lentz 1 Levi Lentz ME 653 10/19/2011 Assignment 3: Parametric study of a system to determine the effect on entropy generation. A Brayton Cycle operates at steady state. The cycle has a pressure ratio of 18:1 with the inlet to the compressor being at ambient conditions (100kPa and 300K). Assume that the entire boundary of the device is at 300K. The temperature of the air at the inlet to the turbine is 700C. Determine the following: a.) what is the net-work output of the device. b.) What is the entropy generation for this cycle? c.) What is the thermal efficiency of the cycle? d.) Do a parametric study in which the pressure ratio is varied from 1:1 to 30:1. What is the trend of the entropy generation as the pressure ratio increases? What happens with the net-heat, work, and BWR? Why would a higher pressure ratio be seen as beneficial? Assume the turbine is fully reversible. 3 T 2 4 1 s Figure 1. The process lines for a completely reversible Brayton Cycle. In order to solve this problem, we must first solve for the temperature of each of the four states shown above. The problem provides two: T1 = 300K (1) T3 = 973K (2) From the Perfect Gas model: P2 k−1 k T2 = T1 ∗ (P ) 1 P4 k−1 k T4 = T3 ∗ (P ) 3 .4 = 300 ∗ (18)1.4 = 685K = T2 (3) .4 = 973 ∗ 1 1.4 (18) = 426K = T4 (4) Lentz 2 a.) To calculate the net-work out: Ẇnet = Ẇturbine − Ẇcompressor = ṁ ∗ (∆hturbine − ∆hcompressor ) (5) Ẇnet = ṁ ∗ cP ∗ (∆Tturbine − ∆Tcompressor ) (6) kg kJ Ẇnet = 5 ∗ 1.005 ∗ (973 − 426 − 300 + 685) = 814.6kW [ ∗ ∗ K = kW] s kg∗K (7) The turbine will have a net-work of 814.6kW. b.) To determine the entropy generation, we need to use the entropy rate equation: Q̇ dS = ṁ ∗ (sin − sout ) + T + Ṡgen dT (8) B Since the turbine is operating at steady state: dS dT =0 (9) Which implies (assuming a constant boundary temperature): ̇ ̇ ̇ Q Q Q Ṡgen = ṁ ∗ (sout − sin ) + Tout − Tin = ṁ ∗ (sout − sin ) + Tnet B B B (10) In order to solve this we need to first use the energy rate equation to determine the net heat of the system: dE dT = 0 = −ṁ(∆h) + Q̇net − Wnet (11) Q̇net = Ẇnet + ṁ ∗ cP ∗ (∆T) = 814.8 + 5 ∗ 1.005 ∗ (426 − 300) = 1448kW (12) Next is to solve for the ∆𝑠 = 𝑠𝑜𝑢𝑡 − 𝑠𝑖𝑛 : T P ∆s = cp ∗ ln ( Tout ) − R ∗ ln ( Pout ) in in (13) Since the entrance and exit pressures are the same: 426 kJ ∆s = 1.005 ∗ ln (300) = .35188 kg (14) Lentz 3 Solving, numerically: 1448 kJ Ṡgen = 5 ∗ .35188 + 300 = 6.586 kgK (15) c.) Determine the thermal efficiency using the following: 𝜂= 𝑊𝑎𝑛𝑡 𝑁𝑒𝑒𝑑 = 𝑊̇𝑡𝑢𝑟 −𝑊̇𝑐𝑜𝑚𝑝 𝑄̇𝑖𝑛 𝑇 −𝑇 426−300 = 1 − 𝑇4 −𝑇1 = 1 − 973−685 = 56.3% 3 2 (16) d.) The parametric study is best done using a program such as excel. The results can be seen following the completion of the problem. Below are the results of the study plotted: Entropy-Generation vs. Pressure Ratio 20 18 16 S-gen (kJ/kgK) 14 12 10 8 6 4 2 0 0 5 10 15 20 25 30 35 Pressure Ratio Figure 2. Entropy Generation vs. Pressure Ratio. The Pressure Ratio appears to be having a strong ability to make the entropy generation decrease. Notice the primary trend of the entropy generation decreases as the pressure ratio increases. This would imply why there is a trend to have a higher pressure ratio in Brayton Cycles. Lentz 4 Efficiency vs. Pressure Ratio 0.7 0.6 Efficiency 0.5 0.4 0.3 0.2 0.1 0 0 5 10 15 20 25 30 Pressure Ratio Figure 3. Efficiency vs. Pressure Ratio. The Pressure Ratio appears to have a drastic effect on the Efficiency. Heat/Work vs. Pressure Ratio 4000 3500 Energy (kW) 3000 2500 2000 Net Work 1500 Net Heat 1000 500 0 0 5 10 15 20 25 30 Pressure Ratio Figure 4. Net-work and net heat vs. Pressure Ratio. Note that the two are converging together. This implies that the engine is getting more efficient as the pressure ratio increases; however the net power output is decreasing. Lentz 5 BWR vs. Pressure Ratio 0.9 0.8 0.7 BWR 0.6 0.5 0.4 0.3 0.2 0.1 0 0 5 10 15 20 25 30 35 Pressure Ratio Figure 5. Back Work Ratio (BWR) vs. Pressure Ratio. The increase of the BWR is to be expected as the pump has to work more to compress the air. It is easy to see from the graphs above why a higher pressure ratio is desired for the Brayton Cycle. Not only does the entropy generation decrease as the ratio increased, but, necessarily, the efficiency increases. The Net Heat of the system also decreases. Unfortunately, as the pressure ratio increases, so does the BWR, decreasing the outputted work. Because of both the desirable and undesirable consequences of increasing the Pressure Ratio, optimization should be performed to determine the proper pressure ratio to use. This would have the optimum efficiency and power output while minimizing the entropy generation, heat into the system, and BWR.